CHEM 1214 Lecture Notes - Lecture 5: Naphthalene, Molality, Colligative Properties
82 views2 pages
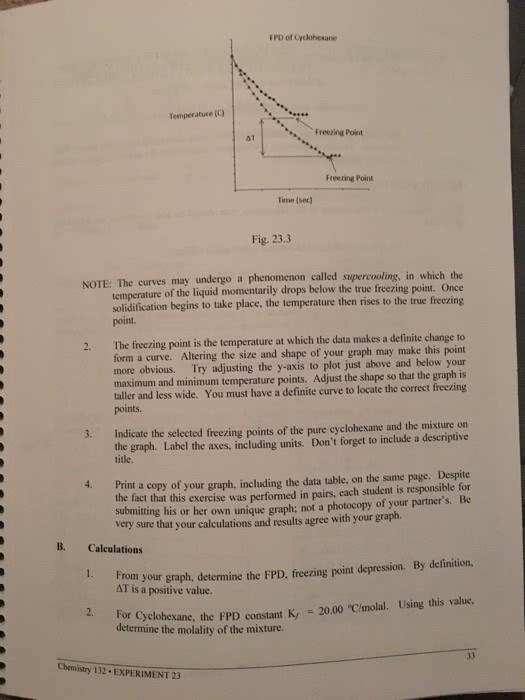
21 Feb 2018
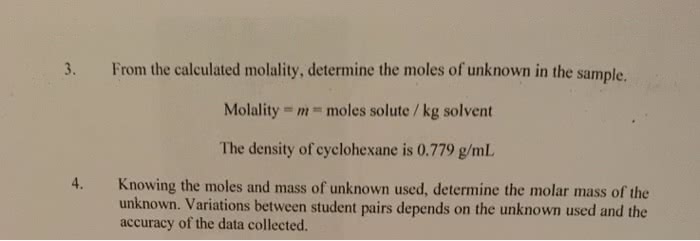
School
Department
Course
Professor
Document Summary
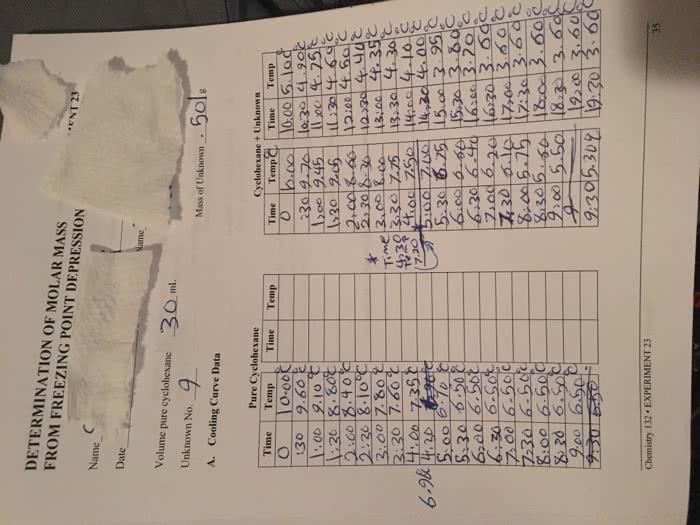
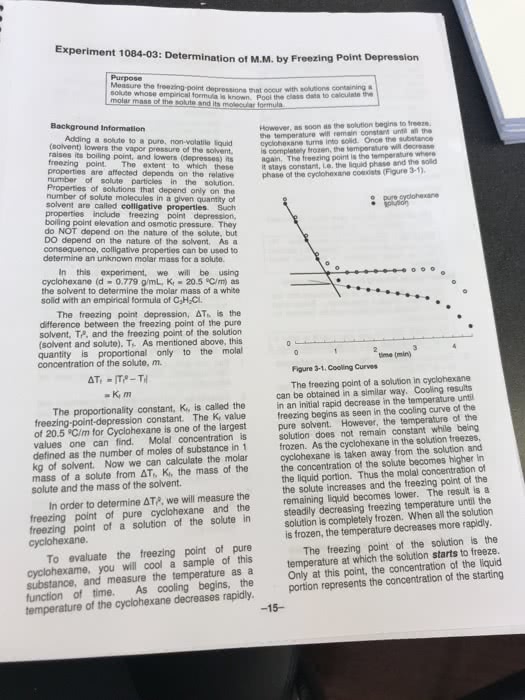
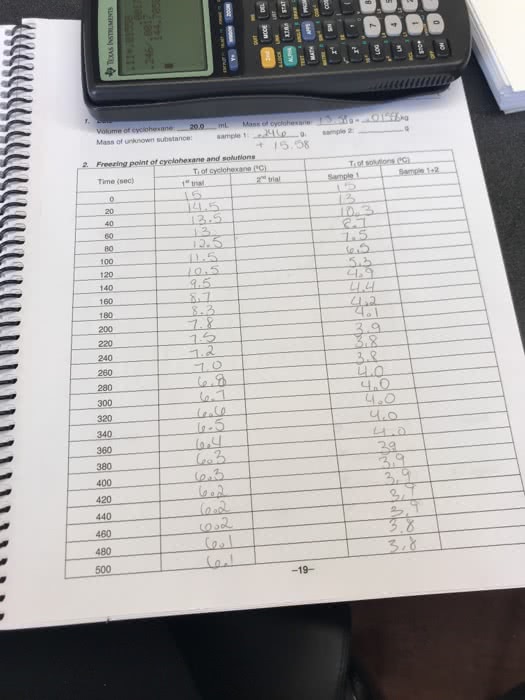
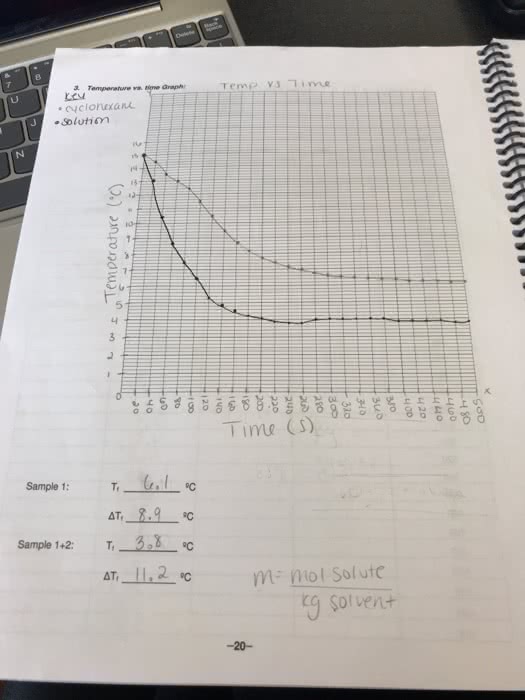
Colligative properties of solutions are defined as those properties that depend on. Introduction: the concentration of the particles in the solution rather than the nature of the particles. If a solute is added to a solvent, the freezing point temperature of the solution lowers, which is relative only to the solvent. The purpose of this lab is to prepare a solution with an unknown solute and. The following experiment involves the use of acetone (via the thermometer), naphthalene as a solvent and measure the change is the freezing point . Based on the change in freezing point and the freezing point constant (kf=6. 9 c/molal) for naphthalene, we will then be able to calculate the molality, and thus the molecular weight of the unknown solute. It is important to be aware that the freezing point of a pure substance is accurately determined by the temperature/time data for the cooling of the substance when it is melted.
Get access
Grade+
$40 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
10 Verified Answers
Class+
$30 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
7 Verified Answers
Related textbook solutions
Chemistry: Structure and Properties
2 Edition,
Tro
ISBN: 9780134293936
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232