1
answer
0
watching
153
views
12 Nov 2019
Determine the pH of the following.
a.) .355 M solution of phenol
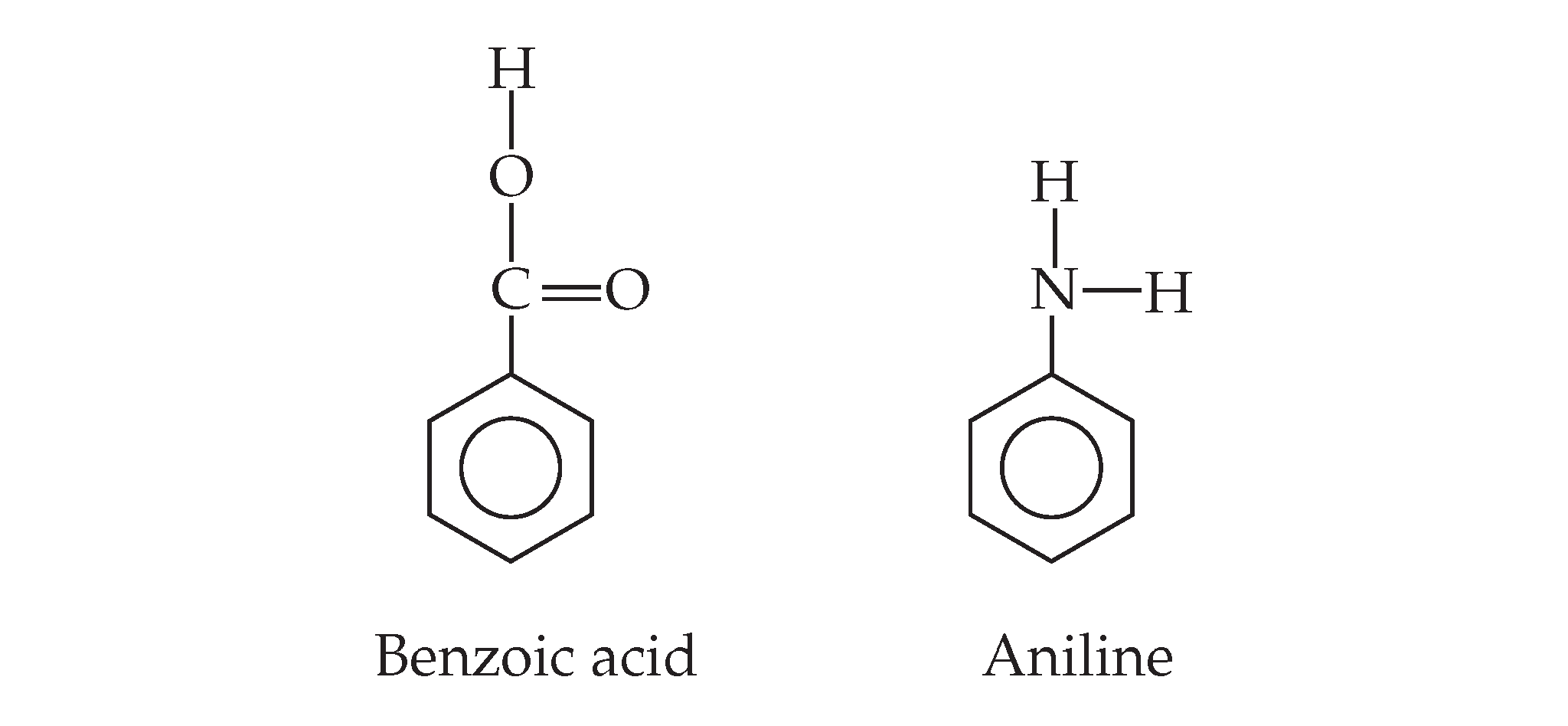
b.) a 6.00*10-3 solution of benzoic acid
c.) a solution made by dissolving .333mol of HNO2 in 500mL of h2o
d.) .500 M solution of aniline
For each acid write the conjugate base then determine the Kb for that base.
Determine the pH of the following.
a.) .355 M solution of phenol
b.) a 6.00*10-3 solution of benzoic acid
c.) a solution made by dissolving .333mol of HNO2 in 500mL of h2o
d.) .500 M solution of aniline
For each acid write the conjugate base then determine the Kb for that base.
Trinidad TremblayLv2
14 Jul 2019