1
answer
0
watching
499
views
11 Dec 2019
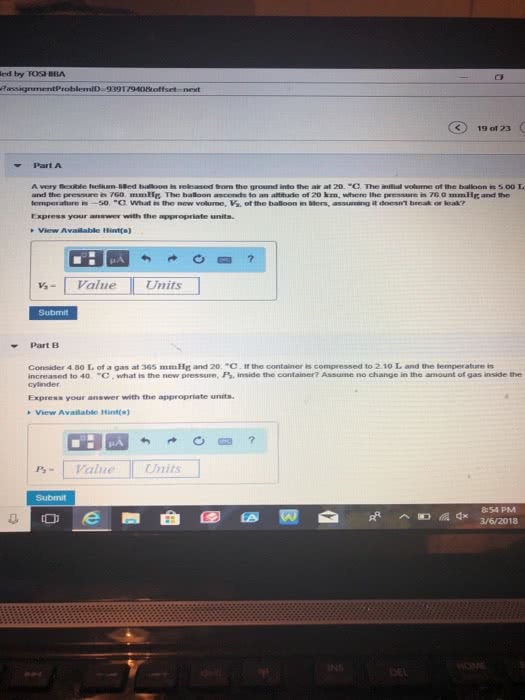
Consider 4.90 L of a gas at 365 mmHg and 20. degrees celisus . If the container is compressed to 3.00 L and the temperature is increased to 40.degrees celisus , what is the new pressure, P2, inside the container? Assume no change in the amount of gas inside the cylinder.
Consider 4.90 L of a gas at 365 mmHg and 20. degrees celisus . If the container is compressed to 3.00 L and the temperature is increased to 40.degrees celisus , what is the new pressure, P2, inside the container? Assume no change in the amount of gas inside the cylinder.
1
answer
0
watching
499
views
For unlimited access to Homework Help, a Homework+ subscription is required.
Hubert KochLv2
12 Dec 2019
Related textbook solutions
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232