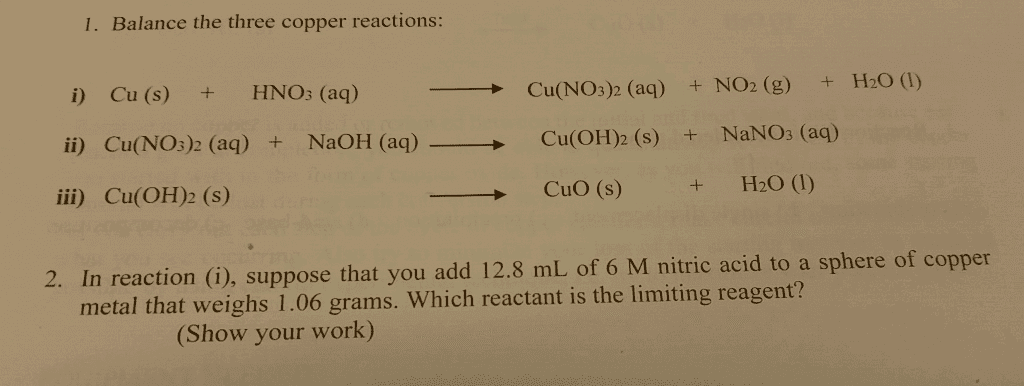0
answers
0
watching
81
views
11 Dec 2019
Suppose that, for the reaction Cu(NO3)2 (aq) + 2NaOH (aq) = Cu(OH)2 (s) + 2NaNO3 (aq) , you have not added enough sodium hydroxide to precipitate all the copper as copper hydroxide. a) How could you tell that not all the copper had precipitated? b) What effect would this have on your final yield?
Suppose that, for the reaction Cu(NO3)2 (aq) + 2NaOH (aq) = Cu(OH)2 (s) + 2NaNO3 (aq) , you have not added enough sodium hydroxide to precipitate all the copper as copper hydroxide. a) How could you tell that not all the copper had precipitated? b) What effect would this have on your final yield?