1
answer
1
watching
323
views
6 Oct 2020
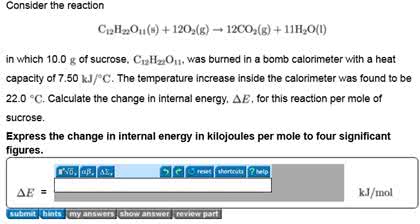
A 1.00 g sample of ordinary table sugar (sucrose, C12H22O11) is burned in a bomb calorimeter. The temperature of 1.50×103 g of water in the calorimeter rises from 25.00 °C to 27.32 °C. The heat capacity of the bomb is 837 J/K, and the specific heat capacity of the water is 4.20 J/g K. Calculate (a) the heat evolved per gram of sucrose and (b) the heat evolved per mole of sucrose.
A 1.00 g sample of ordinary table sugar (sucrose, C12H22O11) is burned in a bomb calorimeter. The temperature of 1.50×103 g of water in the calorimeter rises from 25.00 °C to 27.32 °C. The heat capacity of the bomb is 837 J/K, and the specific heat capacity of the water is 4.20 J/g K. Calculate (a) the heat evolved per gram of sucrose and (b) the heat evolved per mole of sucrose.
Patrick SuarezLv10
12 Nov 2020