CHEM 1040 Study Guide - Final Guide: Intermediate 1, Nucleophilic Substitution, Bromine
59 views6 pages
16 May 2020
School
Department
Course
Professor
Document Summary
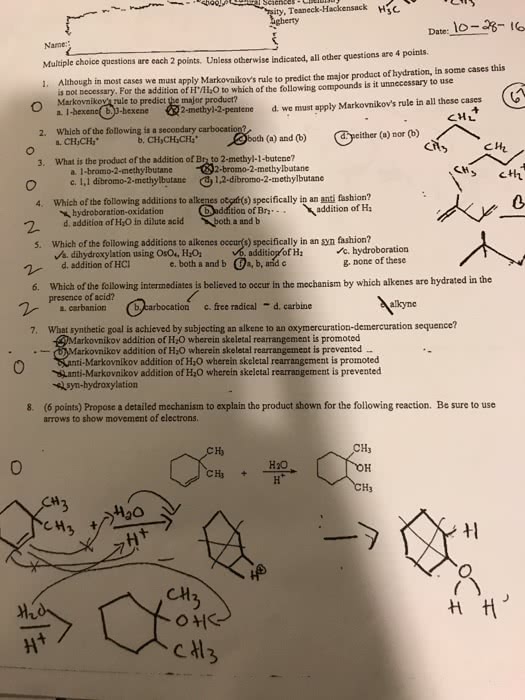
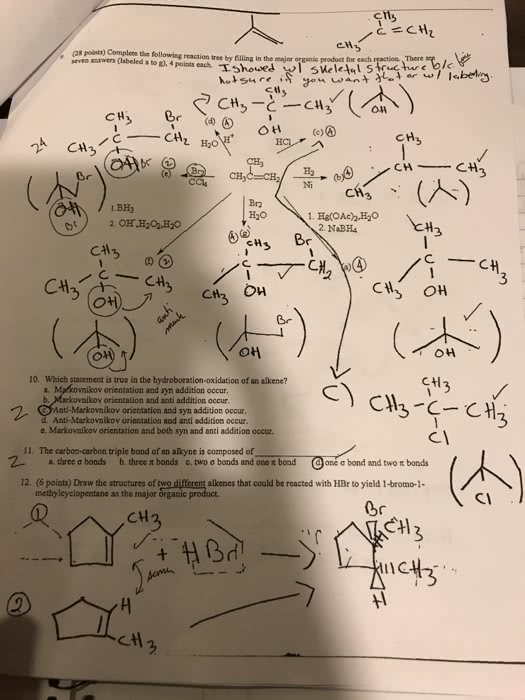
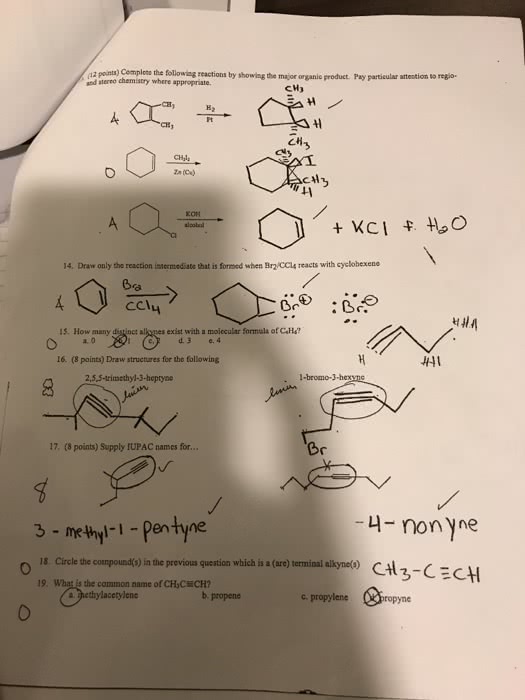
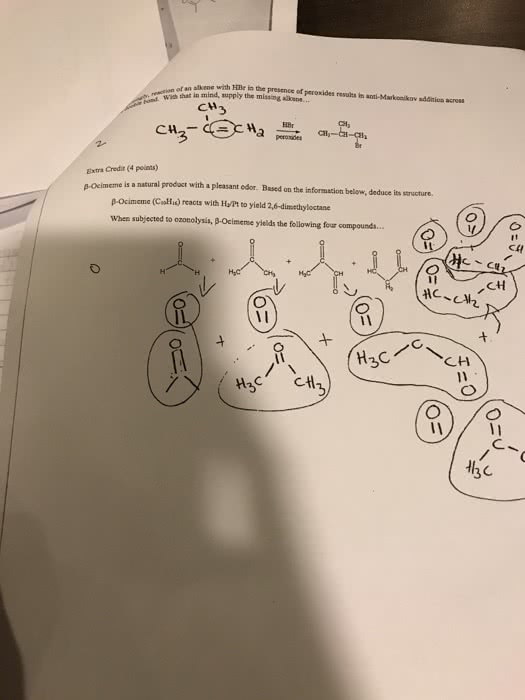
The electrophile, h+, opens up the pi bond of the alkene by bonding with two electrons and the h goes to the side with the most h"s (to produce a methyl group on the end). We now have a secondary carbocation intermediate due to the formal charge left on the carbon atom. The nucleophile, br , is then attracted to the 2o carbocation to complete the structure. Light (or heat) breaks the bromine molecule into two free bromine radicals. One radical removes an h atom from the alkane. The preference is a tertiary hydrogen over a secondary or primary. This produces hbr and a tertiary free radical as an intermediate. The second bromine radical then reacts with the tertiary free radical to produce the major product. 4-methyl-1-cyclohexanol is achiral (no carbon has four different groups due to the symmetry within this molecule subsitutents are directly across from one another).
Get access
Grade+
$40 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
10 Verified Answers
Related textbook solutions
Chemistry: Structure and Properties
2 Edition,
Tro
ISBN: 9780134293936
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232