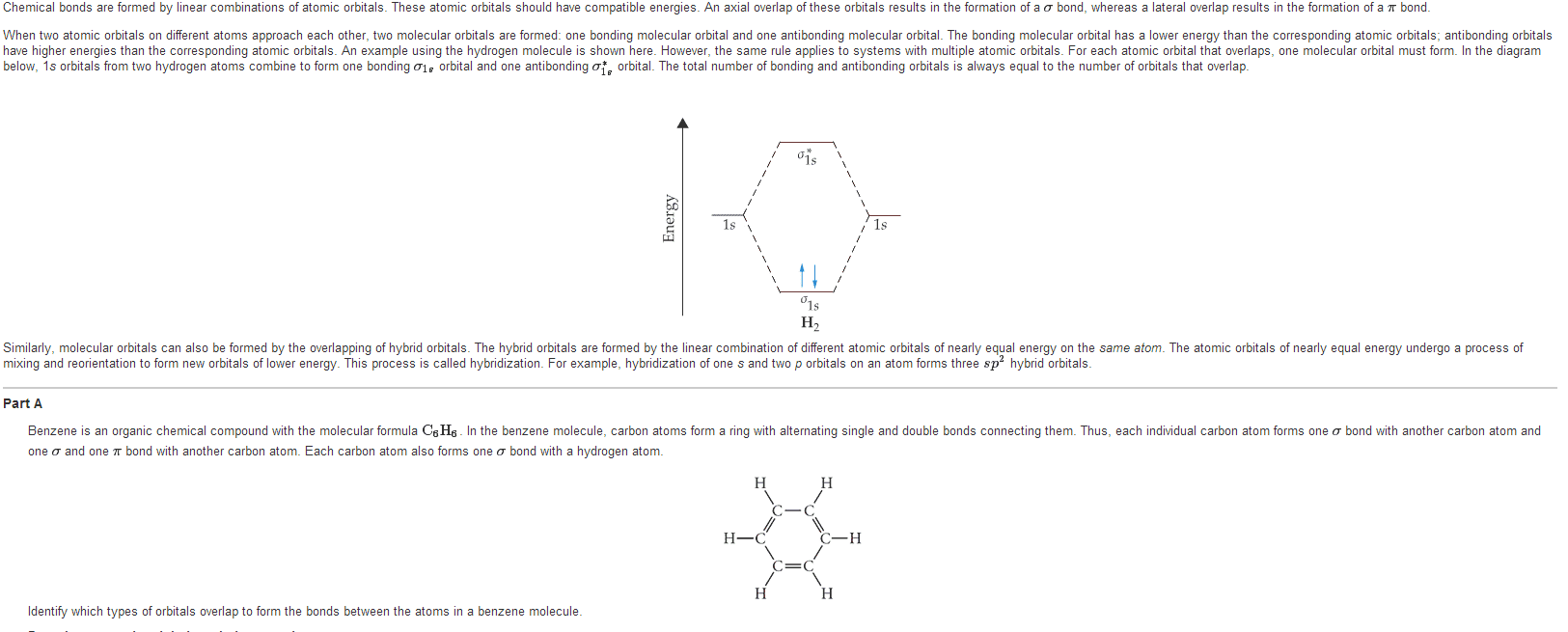
CHEM 322aL Chapter Notes - Chapter 1: Ethylene, Acetylene, Antibonding Molecular Orbital
55 views1 pages
Document Summary
Chapter i: remembering general chemistry: electronic structure and bonding. How the electrons in an atom are distributed. Atomic orbitals (s, p, d, f) have characteristic shapes and energies, they also occupy a characteristic volume of space. Degenerate orbitals have the same amount of energy. The aufbau principle states that an e- always goes into the available orbital with the lowest energy. The pauli exclusion principle states that no more than two e- can occupy each atomic orbital and they must have opposite spin. Hund"s rule states that when there are 2+ atomic orbitals with the same energy an e- will occupy an empty orbital before it will pair up with another e- The octet rule states that atoms are the most stable if the outer shell are filled or contains 8 e- How the structure of a compounded is represented. A radical (free radical) is an atom with a single unpaired e: atomic orbitals.
Get access
Grade+
$40 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
10 Verified Answers
Class+
$30 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
7 Verified Answers
Related textbook solutions
Chemistry: Structure and Properties
2 Edition,
Tro
ISBN: 9780134293936
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232