CHEM 110 Lecture Notes - Lecture 6: Isoelectronicity
49 views1 pages
Verified Note
29 Sep 2018
School
Department
Course
CHEM 110 verified notes
6/24View all
Document Summary
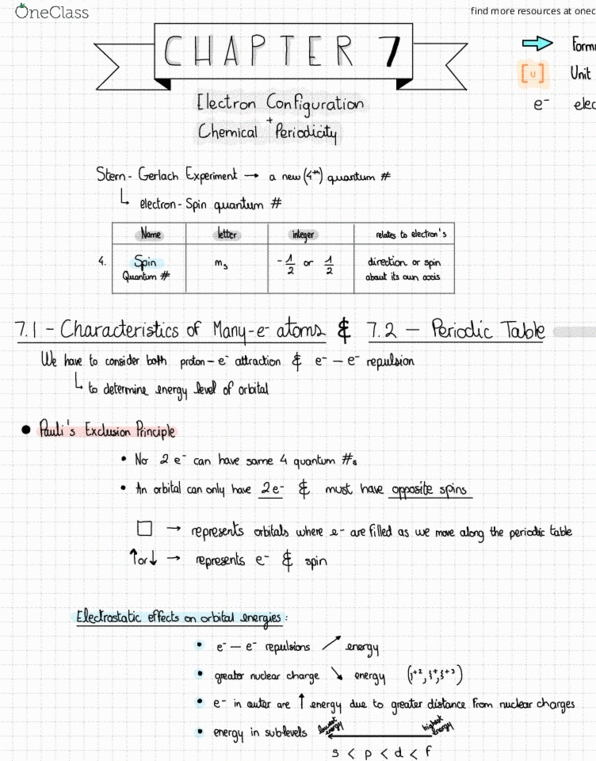
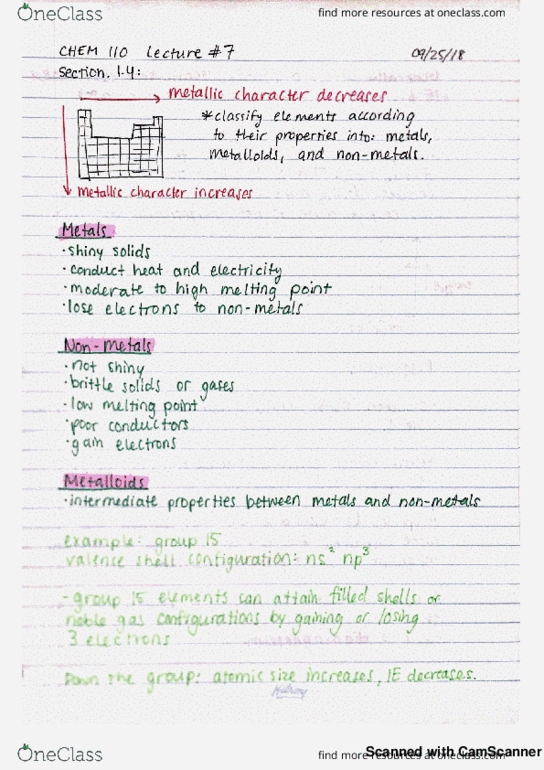
F! valence sub shells t next available sub she " has higher inert at s & p are. G similar e configuration general description : ns similar chemical properties n p. 35 transition elements = elements who have partially filled d orbitals or can make ions with partially filled d orbitals exceptions agg iron: cr, cu. Types of electrons : core e- e that atom has in common with previous noble gas t any completed transition series. Formed e is lost in valence shell for main group = valence e. Isoelectronic same e when an atom & ion have the same e ie :
Get access
Grade+20% off
$8 USD/m$10 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
40 Verified Answers
Class+
$8 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
30 Verified Answers
Related textbook solutions
Chemistry: Structure and Properties
2 Edition,
Tro
ISBN: 9780134293936
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232