CHEM 1A03 Lecture Notes - Lecture 4: Photon, Threshold Energy, Quantum Mechanics
CHEM 1A03 verified notes
4/36View all
Document Summary
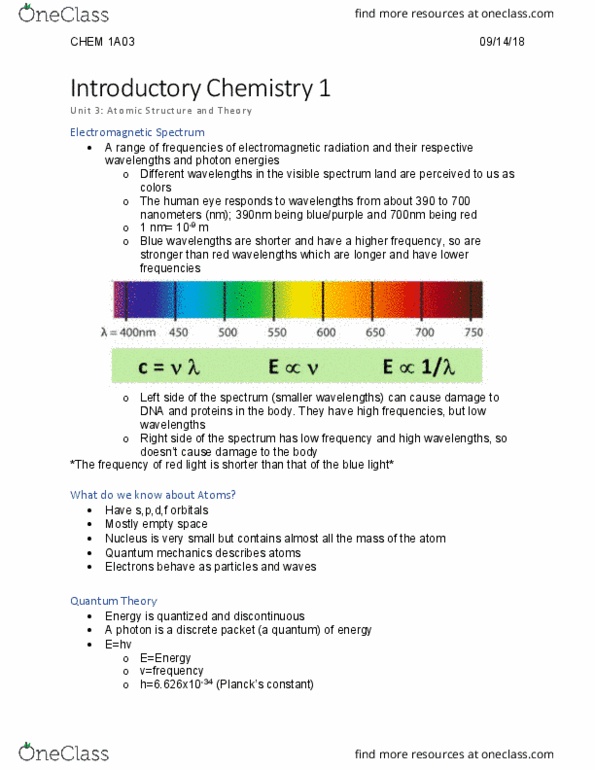
They have high frequencies, but low wavelengths: right side of the spectrum has low frequency and high wavelengths, so doesn"t cause damage to the body. *the frequency of red light is shorter than that of the blue light* What do we know about atoms: have s,p,d,f orbitals, mostly empty space, nucleus is very small but contains almost all the mass of the atom, quantum mechanics describes atoms, electrons behave as particles and waves. Quantum theory: energy is quantized and discontinuous, a photon is a discrete packet (a quantum) of energy, e=hv, e=energy, v=frequency, h=6. 626x10-34 (planck"s constant) 09/14/18: helped in the discovery of the photoelectric effect. The photoelectric effect: albert einstein won the nobel prize for discovering this effect in 1921. I=2xa (x>0: key concepts, shining a light on a metal surface causes electrons to be ejected (called photoelectrons), but only if the light is energetic enough, 1 photon that has enough energy will release only 1 electron.