CHEM 1E03 Lecture Notes - Lecture 7: Sulfuric Acid, Chemical Polarity, Metallic Bonding
CHEM 1E03 verified notes
7/40View all
Document Summary
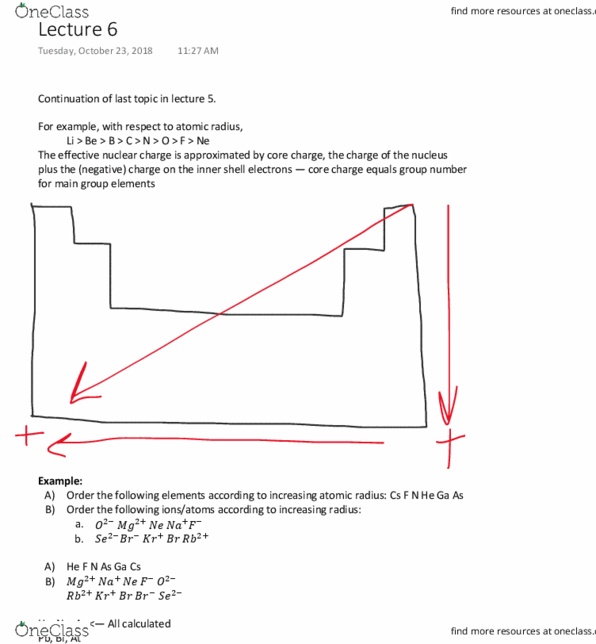
Electrons are bound, in a deep well , to take them out requires energy. To take an e that is originally free is an exothermic rxn. General trends of ea are the same as ie. Exceptions to trend cl has largest magnitude (larger than fluorine - too small repulsion to be largest mag) Noble gases - they dont want the extra e bc they already have filled s and p orbitals - but when you put it there, it gives it more energy, makes it positive - endothermic rxn. When you add es, you get an anion, more favourable ea. So much repulsion in be and mg that its not favourable at all. All the other properties previously discussed were non-interacting atoms (separated atoms in gas phase) Cps depend on number of valence e - s & z eff. Oxides of of 3rd, 4th row elements have a chem. trend.