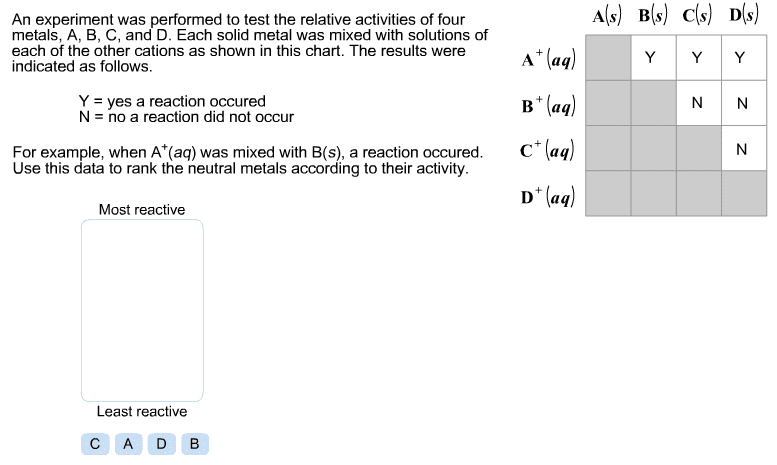
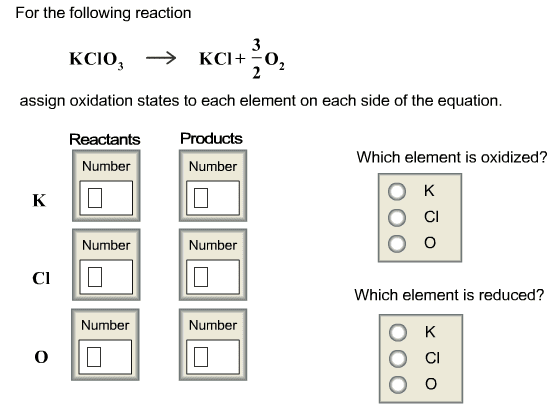
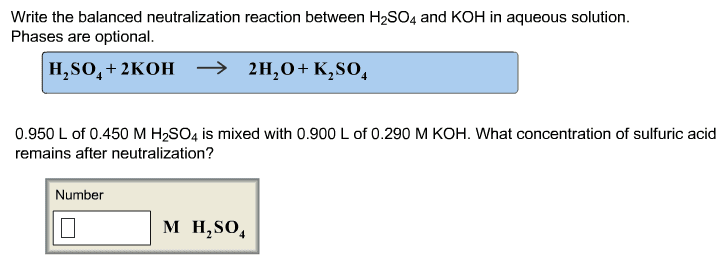
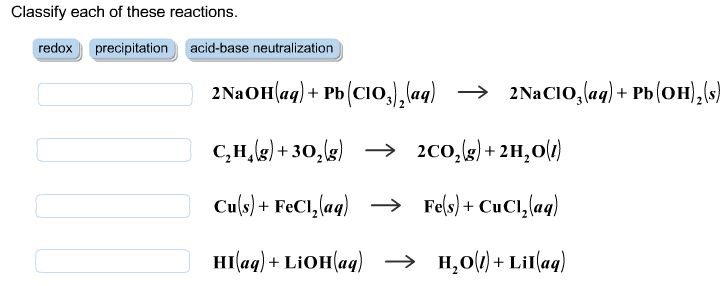CHEM 212 Lecture Notes - Equivalence Point, Sodium Hydroxide, Titration
29 views2 pages
19 Jan 2013
School
Department
Course
Professor
Document Summary
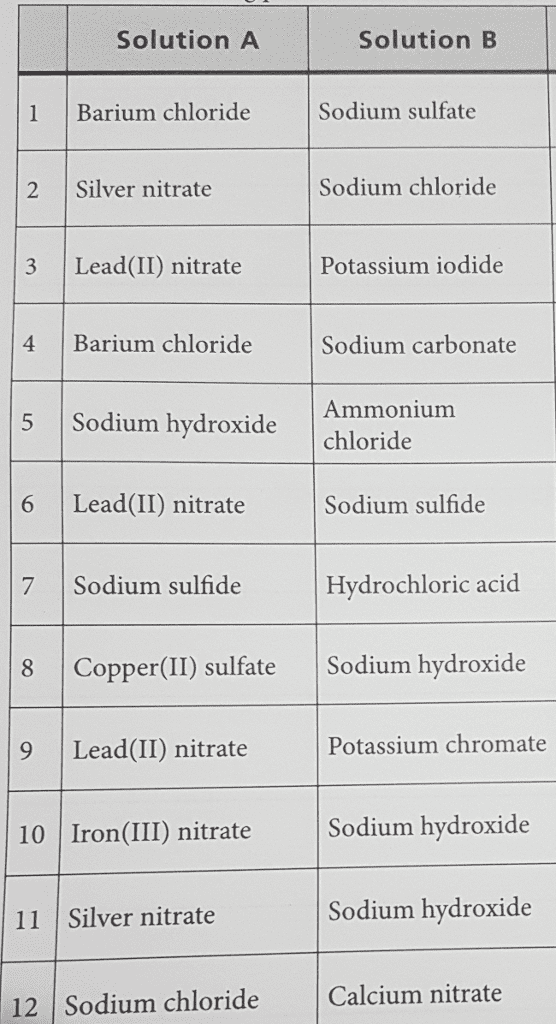
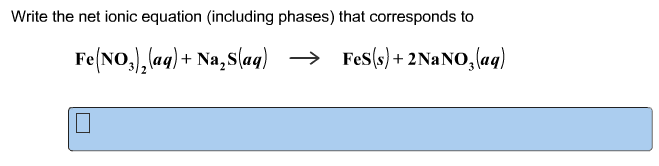
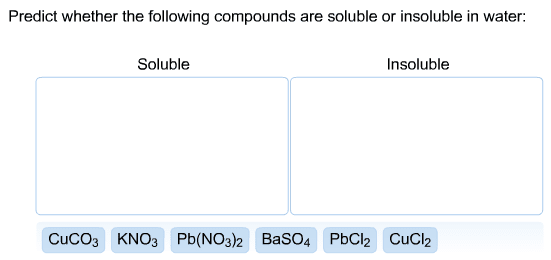
Problem: write the net ionic equation for the reaction that occurs when aqueous solutions of magnesium sulphate (mgso4) and sodium oxalate (na2c2o4) are mixed. Acids are identified by their sour tastes, their ability to react with a variety of metals and carbonate minerals. From a chemist"s standpoint, an acid can be defined as a substance that provides hydrogen ions (h+) in aqueous solutions. A strong acid completely ionizes in a water solution: Hcl (aq) (h20) h+ (aq) + cl- (aq) (aq). A weak acid, on the other hand, does not go towards completion and is also a weak electrolyte. A example is acetic acid: hc2h3o2 (aq) h+ (aq) + c2h3o2. Hcl h+ + cl- : strong electrolyte, strong acid. Ch3cooh h+ + ch3coo- : weak electrolyte, weak acid. H2so4 h+ + hso4- : strong electrolyte, strong acid. From a practical standpoint, a base is bitter in taste and slippery.
Get access
Grade+20% off
$8 USD/m$10 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
40 Verified Answers
Class+
$8 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
30 Verified Answers
Related textbook solutions
Chemistry: Structure and Properties
2 Edition,
Tro
ISBN: 9780134293936
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232