CHEM101 Lecture Notes - Lecture 30: Conjugate Acid, Lewis Structure, Electronegativity
CHEM101 verified notes
30/41View all
Document Summary
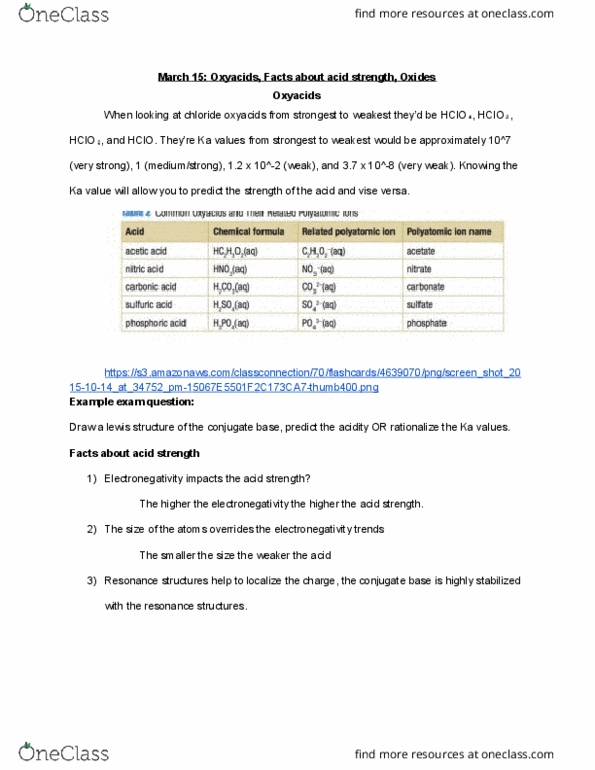
March 15: oxyacids, facts about acid strength, oxides. When looking at chloride oxyacids from strongest to weakest they"d be hclo , hclo , They"re ka values from strongest to weakest would be approximately 10^7 (very strong), 1 (medium/strong), 1. 2 x 10^-2 (weak), and 3. 7 x 10^-8 (very weak). Ka value will allow you to predict the strength of the acid and vise versa. https://s3. amazonaws. com/classconnection/70/flashcards/4639070/png/screen_shot_20. Draw a lewis structure of the conjugate base, predict the acidity or rationalize the ka values. The higher the electronegativity the higher the acid strength: the size of the atoms overrides the electronegativity trends. The smaller the size the weaker the acid: resonance structures help to localize the charge, the conjugate base is highly stabilized with the resonance structures. Metal oxides form with ionic bonding and produce a base in water, the base is oh . Non-metal oxides form with covalent bonding and produce an acid in water.