CHEM101 Lecture Notes - Lecture 39: Intermolecular Force, Identical Particles, Cubic Crystal System
CHEM101 verified notes
39/41View all
Document Summary
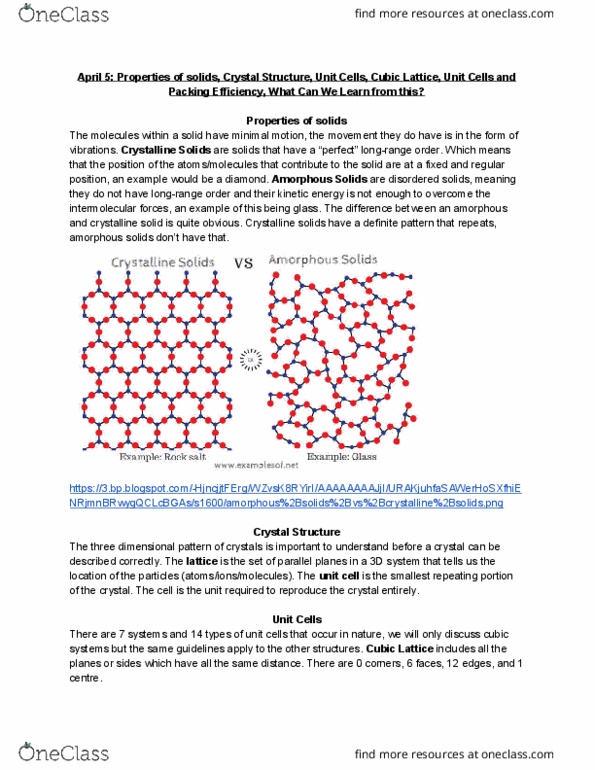
April 5: properties of solids, crystal structure, unit cells, cubic lattice, unit cells and. The molecules within a solid have minimal motion, the movement they do have is in the form of vibrations. Crystalline solids are solids that have a perfect long-range order. Which means that the position of the atoms/molecules that contribute to the solid are at a fixed and regular position, an example would be a diamond. Amorphous solids are disordered solids, meaning they do not have long-range order and their kinetic energy is not enough to overcome the intermolecular forces, an example of this being glass. The difference between an amorphous and crystalline solid is quite obvious. Crystalline solids have a definite pattern that repeats, amorphous solids don"t have that. https://3. bp. blogspot. com/-hjncjjtferg/wzvsk8ryiri/aaaaaaaajji/urakjuhfasawerhosxfhie. The three dimensional pattern of crystals is important to understand before a crystal can be described correctly.