CHEM 1310 Lecture Notes - Jmol, Dihydrogen Cation, Joule
Document Summary
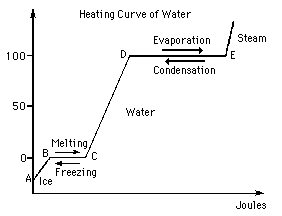
Step 1: melt ice fusion: turns to liquid. Step 2: heat: turns to h2o at 100 c. Phase change from liquid to gas vaporization. Q = quantity of heat: = hfus + h + hvap, =step 1 + step 2 + step 3. Solve: hfus = 6. 02 kj/mol, hvap = 40. 7 kj/mol, look these up in table of values, t = mcspec t, =18. 02g/mol(4. 184 j)(100 k, =7539. 6 j/mol = 7. 54 kj/mol, q = step 1 + step 2 + step 3. =6. 02 kj/mol + 7. 54 kj/mol + 40. 7 kj/mol = 54. 2 kj/mol needed. A substances enthalpy change in going from the standard state to the reference state. The value is 0 if the substance is a pure element in the reference state or it is the ion h+ Standard state - the solid or liquid of the pure element or compound when the pressure is 1 bar - commonly found in table format - 1 bar, 298 k.