I JUST NEED SIMPLE/BASIC ANSWERS NOT LONG ANSWERS
1. Explain how life can be viewed at different levels of biological complexity.
2. Explain how researchers study biology at different levels, ranging from molecules to ecosystems.
3. Distinguish between discovery-based science and hypothesis testing, and describe the steps of the scientific method.
4. Tell whether a given topic or research study is about biology. Tell whether that study is discovery-based or hypothesis testing, or both.
5. If presented with a research study, propose what the Research Question was.
6. If presented with an example, tell whether a statement is a hypothesis, theory or law and why you think so.
Describe the general structure of atoms and their constituent particles.
Relate atomic structure to the periodic table of the elements. List the elements that make up most of the mass of all living organisms.
Explain how a single element may exist in more than one form, called isotopes, and how certain isotopes have importance in human medicine.
Compare and contrast the types of atomic interactions (bonds) that lead to the formation of molecules.
Tell which atoms and how many of each make up a given molecule (formula or diagram).
For a bond in a given molecule, tell whether the bond is covalent, and if it is, tell whether it is polar or nonpolar and why you think so.
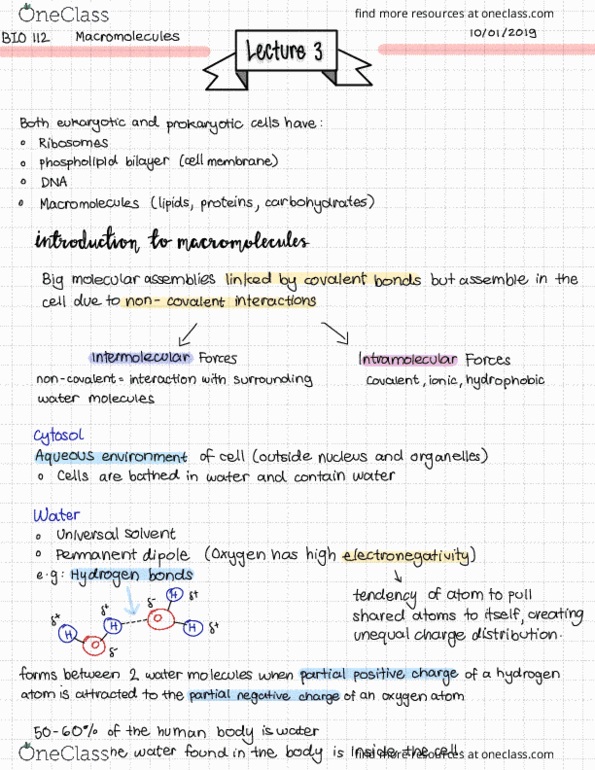
Explain the concept of electronegativity and how it contributes to the formation of polar and nonpolar covalent bonds. Tell whether a given atom is electronegative or not, and why you think so. Label partial charges in a polar bond.
Draw five water molecules and label the atoms and bonds within and between the 5 molecules.
Describe how hydrogen bonding determines many properties of water.
List the properties of water that make it a valuable solvent, and distinguish between hydrophilic and hydrophobic substances.
Explain how water has the ability to ionize into hydroxide ions (OHâ) and into hydrogen ions (H+), and how the H+ concentration is expressed as a solution's pH.
Explain the properties of carbon that make it the chemical basis of all life.
Describe the variety and chemical characteristics of common functional groups of organic compounds.
Diagram how small molecules may be assembled into larger ones by dehydration reactions and how hydrolysis reactions can reverse this process.
List the four major classes of organic molecules and macromolecules found in living organisms
If shown a molecule, tell whether it is organic or not and how you know.
If shown an organic molecule, tell whether it is an amino acid, nucleotide, fatty acid, steroid, monosaccharide or disaccharide and how you know.
Distinguish among different forms of carbohydrate molecules, including monosaccharides, disaccharides, and polysaccharides.
Relate the functions of plant and animal polysaccharides to their structure.
List the several different classes of lipid molecules important in living organisms.
Diagram the structure of a triglyceride and explain how it is affected by the presence of saturated and unsaturated fatty acids. Explain why some fats are solid at room temperature, and others are liquid.
Discuss how fats function as energy-storage molecules.
Apply knowledge of the structure of phospholipids to the formation of cellular membranes.
Describe the chemical nature of steroids and give an example of their biological importance.
Give examples of the general types of functions that are carried out in cells by different types of proteins.
Explain how amino acids are joined to form a polypeptide, and distinguish between a polypeptide and protein.
Describe the levels of protein structure and the factors that determine them. If shown a protein diagram, label parts that represent its levels of structure. Tell what kind of research techniques are used to determine protein structure.
Outline the bonding forces important in determining protein shape and function.
Explain what domains are and their importance in proteins.
Describe the three components of nucleotides.
Distinguish between the structures of DNA and RNA.
Tell how certain bases pair with others in DNA and RNA.
Discuss the 4 major categories of organic macromolecules found in living things, including structure, function and location of each.
Compare and contrast the general structural features of prokaryotic and eukaryotic cells.
Outline the differences in complexity among bacteria, animal, and plant cells.
Describe how a eukaryotic cell can be viewed as four interacting systems: the nucleus, cytosol, endomembrane system, and semiautonomous organelles.
Explain how the proteome underlies the structure and function of cells.
Analyze how cell size and shape affect the surface area/volume ratio.
Identify the location of the cytosol in a eukaryotic cell and list its general functions.
Describe the three types of protein filaments that make up the cytoskeleton. Identify which underlie cilia, flagella, centrioles, the nuclear lamina, and the brush border. Tell which are readily assembled and disassembled. Tell which are involved in various cell shape changes or movements.
Explain how motor proteins interact with microtubules or actin filaments to promote cellular movement.
Describe the structure and organization of the cell nucleus. Describe the structure and function of nuclear pores.
Outline the structures and general functions of the components of the endomembrane system.
Distinguish between the rough endoplasmic reticulum and the smooth endoplasmic reticulum.
Identify three important functions of the plasma membrane.
Tell why mitochondria and chloroplasts are called semi-autonomous organelles.
Discuss the evidence for the endosymbiosis theory.
Outline the structures and general functions of mitochondria and chloroplasts.
Explain how proteins are moved via vesicles through the endomembrane system.
If you are told about the function of a specific protein, tell where and in what type of cell that protein might be found.
Sketch and label a âtypicalâ animal cell and a real animal cell such as a red blood cell or a muscle cell. Tell how it might be different than the âtypicalâ cell in terms of its organelles and shape.
Sketch and label a âtypicalâ plant cell and a real plant cell such as a root or leaf cell. Tell how it might be different than the âtypicalâ cell in terms of its organelles and shape.
Describe the fluid-mosaic model of membrane structure. Tell why membranes are described as âfluid mosaicsâ.
Draw 10 phospholipid molecules as they would be arranged in a bilayer. Label the hydrophobic and hydrophilic parts.
61. Name all the places in a cell where membranes are found.
62. Describe at least three different categories of membrane proteins and tell what they do.
63. Describe what determines how fluid membranes are.
64. Explain how lipids are synthesized at the ER membrane.
65. Explain how transmembrane proteins are inserted into the ER membrane.
66. Compare and contrast diffusion, facilitated diffusion, passive transport, and active transport.
67. Explain the process of osmosis and how it affects cell structure.
68. Outline the functional differences between channels and transporters. Compare and contrast uniporters, symporters, and antiporters.
69. Explain the difference between primary active transport and secondary active transport.
70. Describe the structure and function of the sodium-potassium pump in animal cells and a similar pump in plants and prokaryotes.
71. Describe exocytosis and endocytosis and tell what molecules are moved across the membrane by these.
Discuss membrane transport, including its function and various forms and why all membranes have each form.
73. If a given cellular process is described, tell which form of transport might be involved.
List the different categories of animal tissue, providing general functions and specific examples of each.
Name the various organ systems found in many animals, list the components of each, and describe their general functions.