CHEM 121 Lecture Notes - Lecture 7: Trigonal Bipyramidal Molecular Geometry, Electronegativity, Covalent Bond
CHEM 121 verified notes
7/38View all
Document Summary
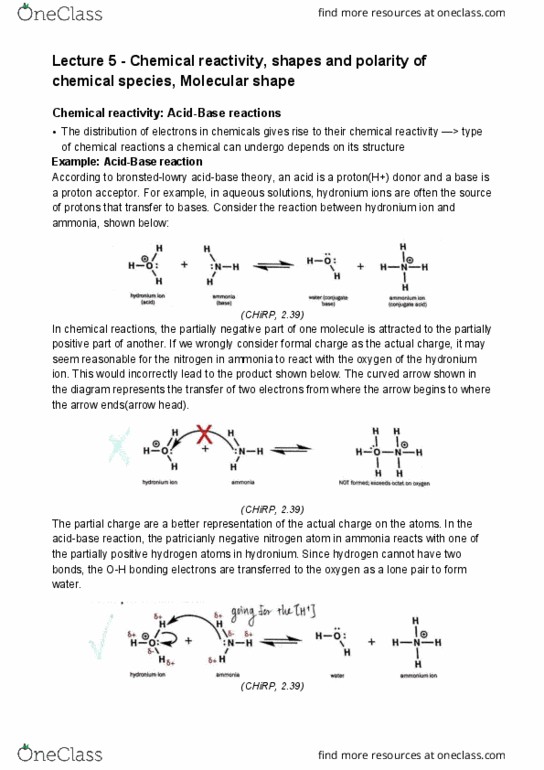
However, the left hand side is a more valid perspective diagram for sulfur tetra uoride because it has 2*90 degree interaction with s-f bonds, while the right hand side diagram has 3*90 degree interaction with the s-f bonds. It is because the lone pairs on cl are less than the lone pairs on f(4<6), there are 4 lone pairs on. Cl, 6 lone pairs on f according to the lewis structure. Because water has a 2 strong lone pairs repulsion on the oxygen atom, causing the molecular shape to bent to form 104. 5 degree, less than its parent shape"s degree which is 109. 5 degree. Tips: check the central atoms > check parent shape of each central atom > check lone pair and bonded pair to determine the molecular shape > calculate the angle between. Step 2: parent shape of the central carbon atoms are both trigonal planar. Step 3: x(bonded atoms) = 3, e(lone pairs) = 0, e+x=3.