CHEM 121 Lecture Notes - Lecture 5: Hydronium, Partial Charge, Lone Pair
CHEM 121 verified notes
5/38View all
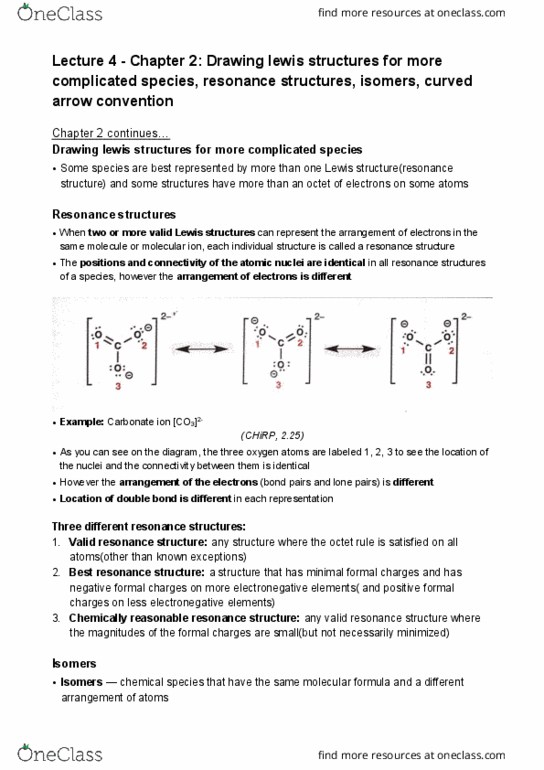
Document Summary
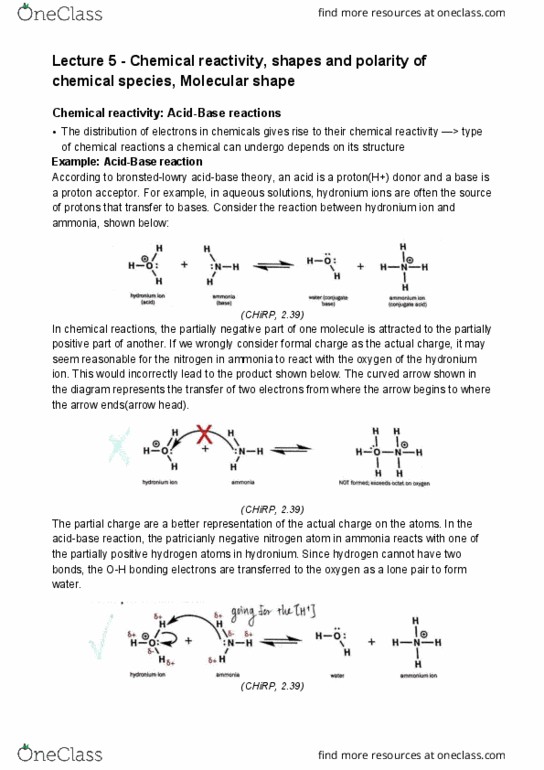
Lecture 5 - chemical reactivity, shapes and polarity of chemical species, molecular shape. Chemical reactivity: acid-base reactions: the distribution of electrons in chemicals gives rise to their chemical reactivity > type of chemical reactions a chemical can undergo depends on its structure. According to bronsted-lowry acid-base theory, an acid is a proton(h+) donor and a base is a proton acceptor. For example, in aqueous solutions, hydronium ions are often the source of protons that transfer to bases. Consider the reaction between hydronium ion and ammonia, shown below: (chirp, 2. 39) In chemical reactions, the partially negative part of one molecule is attracted to the partially positive part of another. If we wrongly consider formal charge as the actual charge, it may seem reasonable for the nitrogen in ammonia to react with the oxygen of the hydronium ion. This would incorrectly lead to the product shown below.