CHEM 121 Lecture Notes - Lecture 22: Covalent Radius, Conjugated System, Molecular Electronic Transition
CHEM 121 verified notes
22/38View all
Document Summary
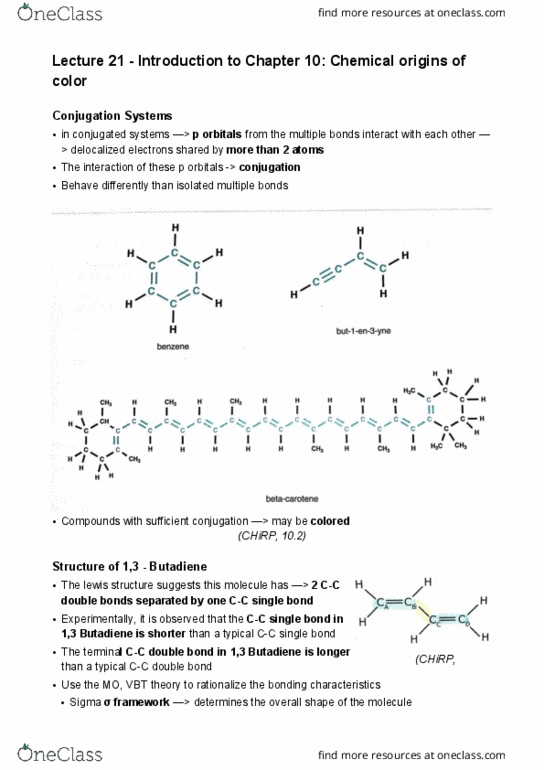
Lecture 22 - chapter 10: conjugated system and organic dye. Exercise: estimate the conjugated chain length required for a molecule to absorb visible light, assume the molecule is 1,3 butadiene. Solution: (chirp, 10. 18: estimate the length of 1,3 butadiene using its double bonds, single bonds and covalent radius. Solution: is a molecule that appears orange absorbing visible light that is higher or lower in energy than a molecule that appears blue. Solution: (chirp, 10. 18) (chirp, 10. 19: calculate the energy difference between the ground state and excited state of a substance that absorbs light at 600nm. Solution: lutein has a structure that is similar to beta-carotene. Its structure is shown below. (chirp, 10. 22) (chirp, 10. 22: compared to beta-carotene, does lutein have a longer box length l, or shorter. Solution: shorter > because there are only 10 c-c double bonds in a row: use this difference in box length to explain why lutein appears yellow and beta carotene appears orange.