PHYS 157 Lecture Notes - Lecture 3: Gas Thermometer, Ideal Gas, Verrazano–Narrows Bridge
PHYS 157 verified notes
3/26View all
Document Summary
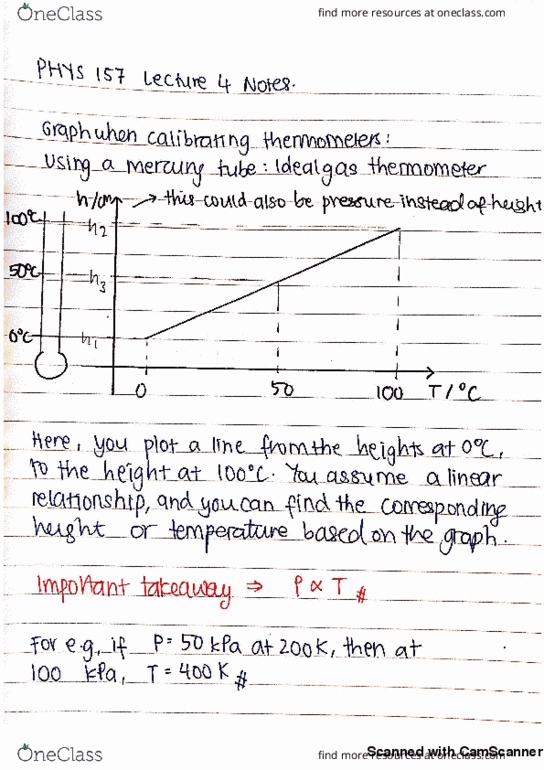
Phys 157 lecture 3 continuation from last class and thermo expansion. Today"s topics: summary of thermometers/temperature characterization: how it works (more quantitative view, celsius versus kelvin: why kelvin, thermo expansion: microscopic to macroscopic. M, b depends on quantities (in celsius units) T-intercept = -b/m = -273 degrees c (universal quantity) Tk = t degrees c + 273 degrees c iclicker questions: at 200k, the pressure of a (nearly) ideal gas in a sealed fixed volume container is 50kpa. The container is now placed in an oven and the pressure is observed to increase to 100kpa. The tem of the oven is: 100k, 200k, 300k, 400k, we need to know the constant of proportionality between t and p to answer this. Answer: d: an ideal gas thermometer is calibrated by placing it in equilibrium with water at its triple point. The same thermometer is placed in equilibrium with another container of water.