PHYS 157 Lecture Notes - Lecture 7: Latent Heat, Heat Capacity, Boiling Point
PHYS 157 verified notes
7/26View all
Document Summary
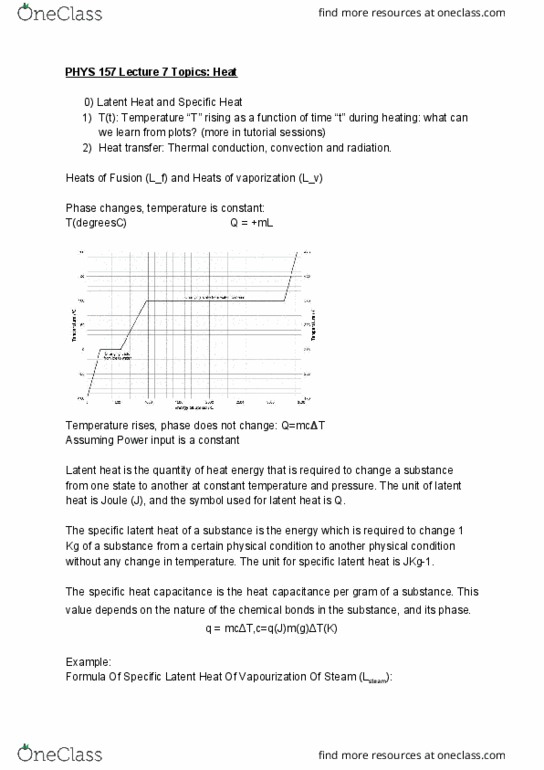
0) latent heat and specific heat: t(t): temperature t rising as a function of time t during heating: what can we learn from plots? (more in tutorial sessions, heat transfer: thermal conduction, convection and radiation. Heats of fusion (l_f) and heats of vaporization (l_v) Latent heat is the quantity of heat energy that is required to change a substance from one state to another at constant temperature and pressure. The unit of latent heat is joule (j), and the symbol used for latent heat is q. The specific latent heat of a substance is the energy which is required to change 1. Kg of a substance from a certain physical condition to another physical condition without any change in temperature. The unit for specific latent heat is jkg-1. The specific heat capacitance is the heat capacitance per gram of a substance.