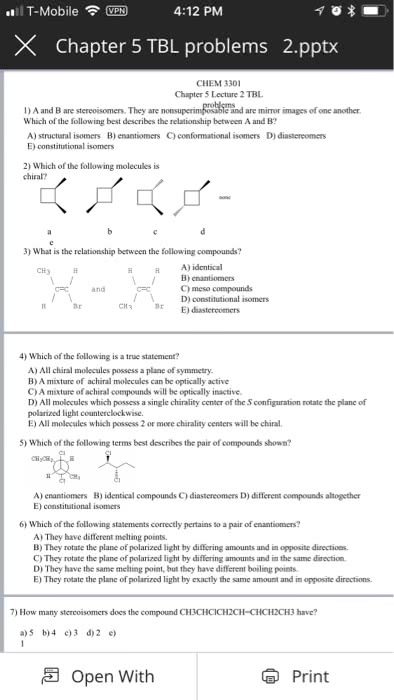
Hi! I am working on a lab for Organic Chemistry and I want to make sure my answers are correct before submitting it!
Thank you in advance!
Stereoisomerism: A Model Exercise
In this experiment you will construct models with your molecular model set that illustrate the concepts of chirality, chiral center (stereogenic center, asymmetric carbon atom), enantiomers, diastereomers, and meso forms. You will also learn about two conventions, R-S and Fischer, for designating the configurations of chiral molecules.
You will be asked several questions related to each construction exercise. Record the answers and then when you have finished all of the exercises, take the assessment listed in this folder. The questions will be repeated so that you can submit your answers electronically for grading.
Chiral centers
Procedure: Construct a model* in which a tetrahedral (sp 3) carbon atom (black) has four different model atoms attached to it. Use the light blue ball, red, blue and, green polyhedrons to represent four different atoms or groups attached to the central atom (black).
1. Does the model have a plane of symmetry?
a. Yes
b. No
A carbon atom that has four different groups attached to it is a chiral center, or an asymmetric carbon atom. The carbon marked with a # in 3-methylhexane is a chiral center.
2. What groups are attached to the chiral center in 3-methylhexane?
a. Hydrogen
b. Methyl
c. Ethyl
d. n-propyl
e. n-butyl
Replace the green atom (or group) in your model with a second red atom. Now two of the groups attached to the carbon atom are identical:
3. Does the model now have a plane of symmetry?
a. Yes
b. No
4. �� Describe it.
a. Plane through the light blue ball, black polyhedron and blue polyhedron
b. Plane through a red polyhedron, black polyhedron and blue polyhedron
c. Plane through the light blue ball, black polyhedron and red polyhedron
d. Plane through a red polyhedron, black polyhedron and red polyhedron
Draw structural formulas for the following compounds, and mark any chiral centers (asymmetric carbons) with an asterisk:
l-bromobutane, 2- bromobutane, 1,2-dibromobutane,
1,3-dibromobutane, l, 4-dibromobutane, 2,3-dibromobutane.
5. Which of the compounds contain chiral centers?
a. 1-bromobutane
b. 2-bromobutane
c. 1,2-dibromobutane
d. 1,3-dibromobutane
e. 1,4-dibromobutane
f. 2,3-dibromobutane
Chirality and Enantiomers
A center of chirality (from the Greek cheir, hand) imparts the property of handedness to a molecule. In this part of the experiment, the left- or right- handedness of molecules with a chiral center will be illustrated with models.
A molecule is said to be chiral (that is, to have the property of handedness) if its mirror image is not superimposable. The mirror image of a left hand, for example, is a right hand. A molecule that is achiral has a mirror image that is superimposible. We shall see that any molecule with a plane of symmetry is achiral.
Procedure: Reconstruct the original model (carbon (black) with light blue ball, red, blue and, green polyhedrons attached). Set the model on the desktop so that the substituent light blue ball atom points toward the ceiling.
Looking down on the model and proceeding clockwise from the green atom, record the colors of the three atoms that rest on the desktop.
Now construct a second model that is the mirror image of the first, and place it on the desktop with the light blue ball atom up:
6. In which direction, clockwise or counterclockwise, must you proceed in order to list the same sequence of colors of the three atoms resting on the desk's surface?
a. Clockwise
b. Counterclockwise
Try to superimpose the two models.
7. The models are
a. Superimposable
b. Not superimposable
The two models that you have just constructed represent chiral molecules- they lack a plane of symmetry and have mirror images that are notsuperimposable. Two substances, the molecular structures of which are related as an object and its nonsuperimposable mirror image are called enantiomers. They differ from each other only in properties that have a direction or "handedness," such as, for example, the direction(clockwise or counterclockwise) in which they rotate a beam of plane-polarized light. Because of this latter property, such substances are sometimes called optical isomers. They are optically active.
Now we will examine the consequence of having at least two identical atoms or groups attached to a tetrahedral carbon atom.
Replace the green atom in each model with a red atom, so that each model has two identical groups attached to the central carbon atom:
8. Are the models still mirror images?
a. Yes
b. No
9. Does either of the models have a plane of symmetry?
a. Yes
b. No
10. Are the models superimposable?
a. Yes
b. No
11. Do the models represent identical molecules or different molecules?
a. Identical
b. Different
Place each model on the desk so that the light blue ball substituent points up.
12. To define the same sequence of colors for the three atoms resting on the desk top, must you proceed
a. Clockwise
b. Counterclockwise
c. Either way
13. Are the models chiral (handed)?
a. Yes
b. No
The models you have just studied represent achiral molecules. Their mirror images are identical. They are optically inactive. Any molecule that has a plane of symmetry is achiral.
Diastereomers and Meso Forms
For any molecule that has two or more chiral centers, it is possible to have stereoisomers that are not mirror images. Stereoisomers that are not related as enantiomers are diastereomers. Diastereomers differ in all properties, chiral and achiral.
Procedure: Construct a model with four different groups (light blue ball, blue, red, and green) attached to a central carbon atom (black). Construct another model identical to the first. (Be sure they are identical by making sure the models are superimposable.) Now remove the green substituent from each model and connect the two carbon atoms with a bond. Use this model with the two stereogenic centers for the next series of questions (14 -26).
14. How many chiral centers (asymmetric carbons) does this model have?
a. 1
b. 2
c. 3
d. 4
e. 5
Note that there are four different groups attached to each chiral center and that each chiral center has the same four groups attached.
15. Does the model have a plane of symmetry in any of its conformations?
a. Yes
b. No
Construct the mirror image of the first model.
16. Is the mirror image identical to or different from the first model?
a. Identical
b. Different
17. What term describes the two models?
a. Enantiomer
b. Diastereomer
18. Is each model chiral or achiral?
a. Chiral
b. Achiral
Now interchange a red and blue atom on the same carbon in one of the models.
19. Are the models identical or different now?
a. Identical
b. Different
20. Are they mirror images (enantiomers)?
a. Yes
b. No
21. Are they stereoisomers?
a. Yes
b. No
22. What term describes the two models?
a. Enantiomer
b. Diastereomer
Carefully examine the conformations of the model in which you interchanged the red and blue atoms.
23. Does the model have a conformation with a plane of symmetry?
a. Yes
b. No
24. Would the mirror image of this model be identical (superimposable) or different from the model itself?
a. Identical
b. Different
Verify your prediction by constructing the mirror-image model.
25. This model is
a. Chiral
b. Achiral
26. Would a molecule corresponding to this model be optically active?
a. Yes
b. No
The last model studied here represents a meso form. The model possesses two chiral centers, but they are of equal and opposite chirality. This situation arises when a molecule has two identical chiral centers. Because the molecule has a readily accessible conformation with a plane of symmetry, it is achiral and optically inactive.
Tartaric acid is a molecule that corresponds to the models constructed in this section of the experiment.
Tartaric Acid
It exists in three forms; two are optically active enantiomers, and the third is an optically inactive meso form that is a diastereomer of the optically active forms. ,
27. Draw Newman projection formulas (looking at the bond between C-2 and C-3) for the three tartaric acids. Label pairs of enantiomers and diastereomers, as well as the meso form.
When a molecule has two different chiral centers, it may exist in four optically active forms (two pairs of enantiomers). To illustrate this with the models you just used, replace one of the colored atoms on one of the carbon atoms with a black atom. There are now four distinct ways of constructing the models: two pairs of enantiomers. Construct the two pairs of enantiomers.
28. What name is given to a pair of molecules consisting of one molecule from each of the two pairs of enantiomers you just constructed?
a. Enantiomers
b. Diastereomers
c. Meso forms
The R-S Convention
The letter R (from rectus, right) or S (from sinister, left) is used to designate the configuration at a chiral center. The four atoms or groups attached to the chiral center are arranged in a priority order according to atomic number: the higher the atomic number, the higher the priority. If two atoms have the same atomic number, we move to the next atoms out from the chiral center, or even further, until we observe a difference in atomic number. We then view the molecule from the side opposite the group with the lowest priority. If the remaining three groups in order from highest to lowest priority form a clockwise array, the configuration is R; if they form a counterclockwise array, the configuration is S.
Procedure: Construct a model of 2-chlorobutane.
29. Which carbon in the chain is a chiral center?
a. 1
b. 2
c. 3
d. 4
There are four groups attached to this chiral center
.
30. Which group has the highest priority?
a. Cl
b. Methyl
c. Ethyl
d. H
31. Which group has the lowest priority?
a. Cl
b. Methyl
c. Ethyl
d. H
32. What is the priority order of the other two groups?
a. Ethyl > Methyl
b. Methyl> Ethyl
Set the model on the desktop so that it can be viewed from the side opposite the hydrogen. Put the chlorine atom at the top.
33. When viewing the model, the three remaining groups in priority- order sequence form a
a. Clockwise array and the model has a R configuration.
b. Counterclockwise array and the model has a R configuration.
c. Clockwise array and the model has a S configuration.
d. Counterclockwise array and the model has a S configuration.
Interchange any two groups attached to the chiral center.
34. What configuration does the model have now?
a. The same as before.
b. The opposite configuration.
Note that to change configuration we must disconnect and remake bonds, whereas we can change conformation by rotating groups around single bonds.
Fischer Projection Formulas
It is sometimes convenient to have a two-dimensional representation for three-dimensional molecules, particularly when we are studying stereoisomerism. One common convention, devised by the German organic chemist Emil Fischer and named after him, is described in this section
Procedure: Construct a model of an asymmetric carbon atom [with a black, a blue, a green, and a red ball attached] that corresponds to the following three-dimensional drawing:
Set the model on the desk as shown. With your left hand, tip the model to the left so that only the red and green balls rest on the table. Viewed from the top, the black and blue balls project toward you (left and right, respectively).
The convention for Fischer projection formulas is as follows: The asymmetric carbon lies in the plane of the page or paper, horizontal groups come out of the plane of the paper toward the viewer, and vertical groups recede behind the paper away from the viewer.
Save the model you have just constructed for comparison with other models that you will construct.
Let us first consider the effect of interchanging any two groups in a Fischer projection formula. Your model corresponds to formula A below:
Consider formula A', with groups blue and green interchanged. Construct a model corresponding to A'.
35. Is A' identical to A, or is it the enantiomer of A?
a. Identical
b. Enantiomer
Now construct a model corresponding to A" (like A, but with green and red interchanged).
36. Is A" identical to A, or is it the enantiomer of A?
a. Identical
b. Enantiomer
37. What generalization can you make about the interchange of any two groups in a Fischer projection formula?
a. The new molecule is an enantiomer of the first.
b. The molecule is identical to the first.
38. If you were to make an even number of group interchanges in a Fischer projection formula, would you obtain the original molecule or its enantiomer?
a. Original molecule
b. Enantiomer of the original model
39. Do the following Fischer projections represent the same molecule or enantiomers?
a. Same molecule
b. Enantiomers
Rotation of a Fischer projection formula in the plane of the paper also affects the structure it represents.
.
Consider formula B, which corresponds to the Fischer projection formula of A rotated 90° clockwise in the plane of the paper. Build a model of formula B. (Remember Fischer Projection Formulas are two dimensional diagrams written on paper of three dimensional molecules.)
40. What minimum number of group interchanges in the model are necessary to convert formula B back to formula A?
a. Zero
b. One
c. Two
d. Three
41. Is model B identical to model A, or is it the enantiomer of model A?
a. Identical
b. Enantiomer
Formula C results from a 90° counterclockwise rotation in the plane of the paper of the Fischer projection formula of A. Build a model of formula C.
42. Is model C identical to model A, or is it the enantiomer of A?
a. Identical
b. Enantiomer
Formula D results from a 180° rotation in the plane of the paper of the Fischer projection formula A . Build a model of formula D.
43. What minimum number of group interchanges are necessary to convert model D back to model A?
a. Zero
b. One
c. Two
d. Three
44. Is model D identical to model A, or is it the enantiomer of A?
a. Identical
b. Enantiomer
45. What generalization can you make about the rotation of a Fischer projection formula in the plane of the paper?
a. 90° rotation results in an enantiomer, 180° rotation results in the identical molecule.
b. 180° rotation results in an enantiomer, 90° rotation results in the identical molecule.