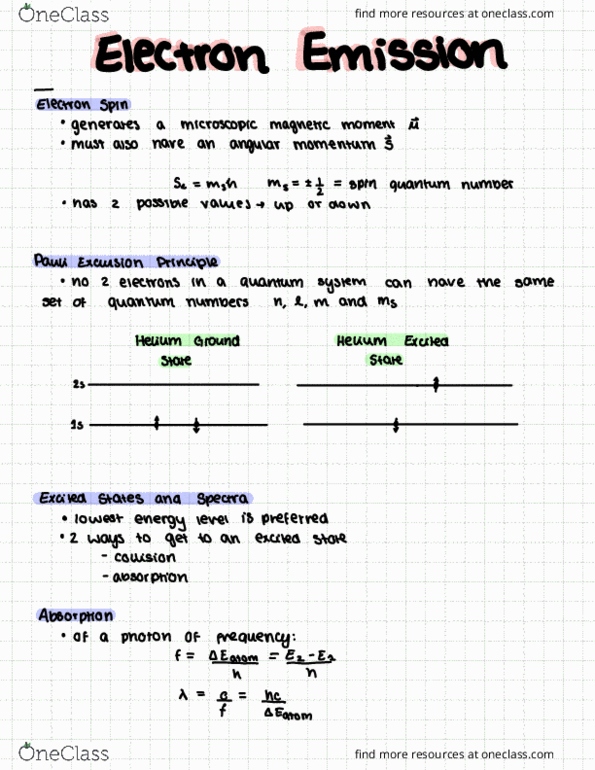
PHYA22H3 Lecture Notes - Lecture 23: Emission Spectrum, Mass Spectrometry, Work Function
PHYA22H3 verified notes
23/26View all
Document Summary
Physics continuous spectrum - light is emitted at every possible wavelength. Higher perfectly absorbing and temp higher radiated emitting intensity by blackbody object. T discharge in electrodes tube a glass tube with low pressure to emit a certain color. Faraday "s gas sealed metal causes gas can create an emission spectrum and an abortion spectrum that will look opposite from emission. Isotopes same element with different amount of electrons ( and. May by have mass spectrometer different nuclear properties. Uv photoelectrons form a current between light cathode hits and cathode anode photoelectrons depends are on only metal emitted if threshold frequency is met work function minimum. Stopping potential current energy reaches needed to thee an electron from a metal. Planck "s constant photons are emitted and absorbed. When photons are absorbed by a completely it metal or not delivers at its all entire energy to one electron. More intense light more photons of same energy threshold frequency fo = eof.