CHM135H1 Lecture Notes - Lecture 2: Electromagnetic Spectrum, Work Function, Kinetic Energy
CHM135H1 verified notes
2/38View all
Document Summary
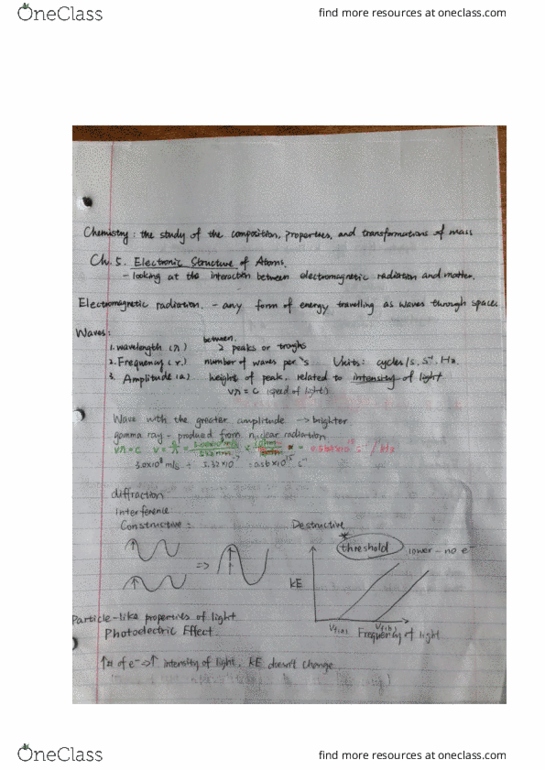
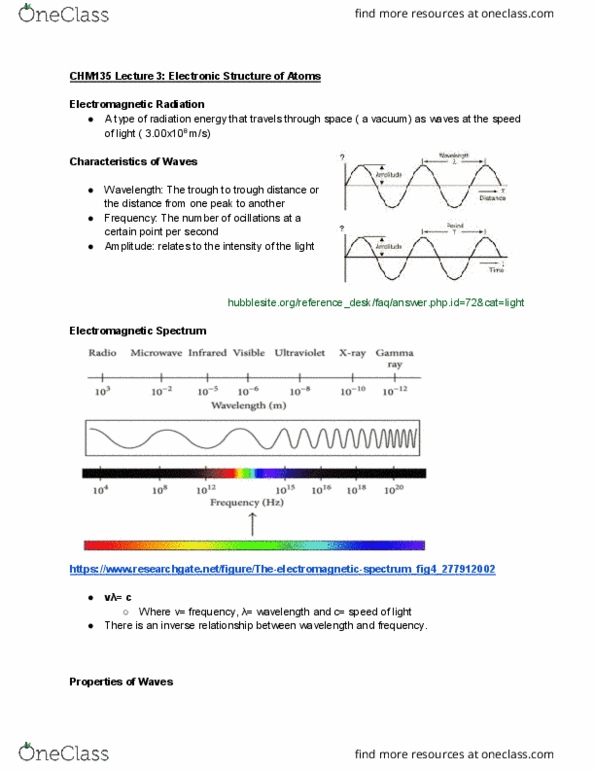
Chemistry: the study of composition, properties, and transformations of matter. All travel at the speed of light (c = 3. 00 * 108 m/s) Characterized by: wavelength ( ): distance between two peaks, frequency ( ): number of waves per second that passes through a given point (units used: cycles per second, s-1, hz, better to use s-1 when solving problems. Wavelength and frequency are inversely proportional: amplitude (a): height of peak, amplitude increases as light intensity increases. Matter: discrete particles with determinate mass, position, etc. Each metal has a unique threshold frequency to eject electrons. Number of ele(cid:272)t(cid:396)o(cid:374)s i(cid:374)(cid:272)(cid:396)ease with f(cid:396)e(cid:395)ue(cid:374)(cid:272)y, (cid:271)ut thei(cid:396) ki(cid:374)eti(cid:272) e(cid:374)e(cid:396)gy does(cid:374)"t. Ephotons = h = hc/ , h = pla(cid:374)(cid:272)k"s (cid:272)o(cid:374)sta(cid:374)t, 6. 6(cid:1006)6 * (cid:1005)(cid:1004)-34 j. Electrons needs enough energy to overcome binding energy to the metal to be ejected. The minimum energy needed is called work function, h = + keelectron. As we increase frequency, we increase kinetic energy of electrons.