CHM135H1 Lecture : Notes taken during lecture

90
CHM135H1 Full Course Notes
Verified Note
90 documents
Document Summary
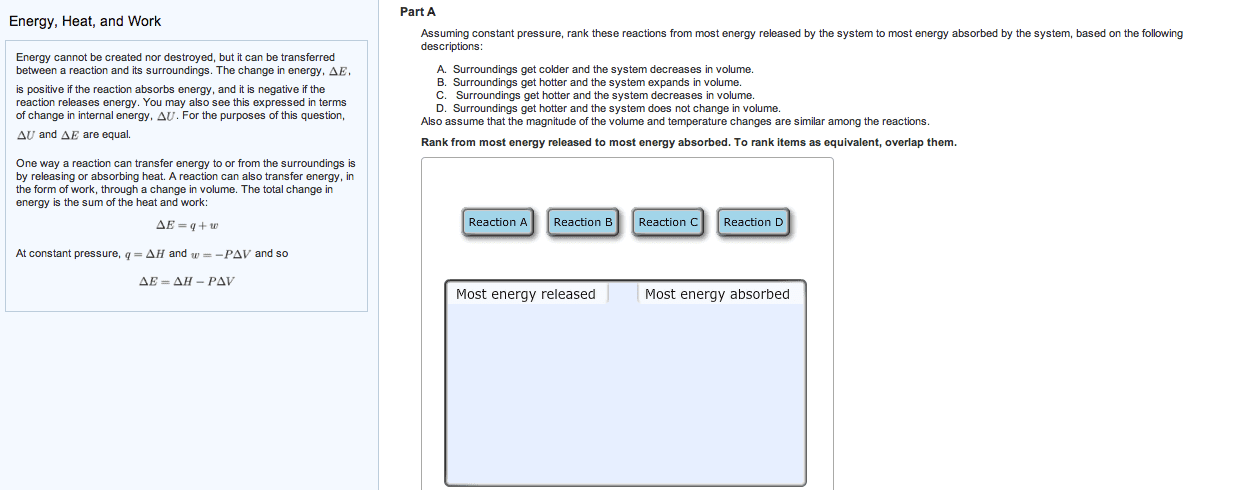
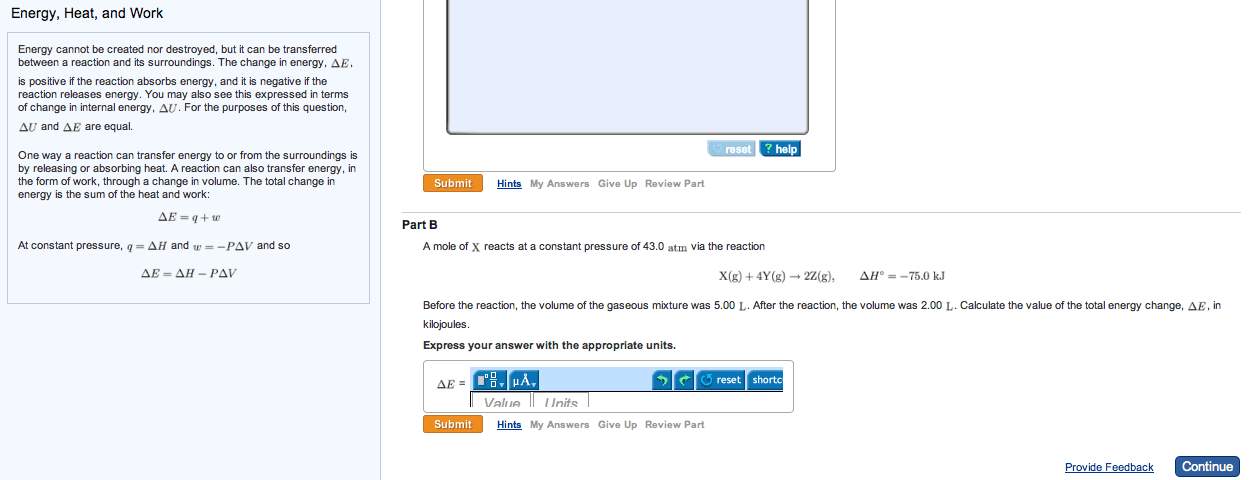
3 q represents heat, or the transfer of thermal energy, which is another type of kinetic energy restating delta e = -pdeltav plus q, which q being equal to delta e plus p delta v common kinds of reactions. The addition sign is circled because the negative sign for work has already been included. A change in volume from 0. 274 l to 448l. W = -p times delta v = -(1atm)(448l-0. 274l) = -447. 7 latm times (101j/latm)(kj/1000j) = -45. 2kj. Negative sign means energy lost, leaving system, so work is done on surroundings. W = -p times delta v = -1atm(5. 6l)(101j/latm) = -566j. Delta e = q + w = -(484kj) + (-566j) = -484. 6kj. Ex. q of surr = q of water = c of water times mass times change in temperature = 4. 18j/gc (250g)(7. 48c)(kj/1000j) = 7. 82 kj. Q of rxn = - q of surr = -7. 82kj. Extensive because enthalpy differs for 1 mole of reactants compared to 5 moles.