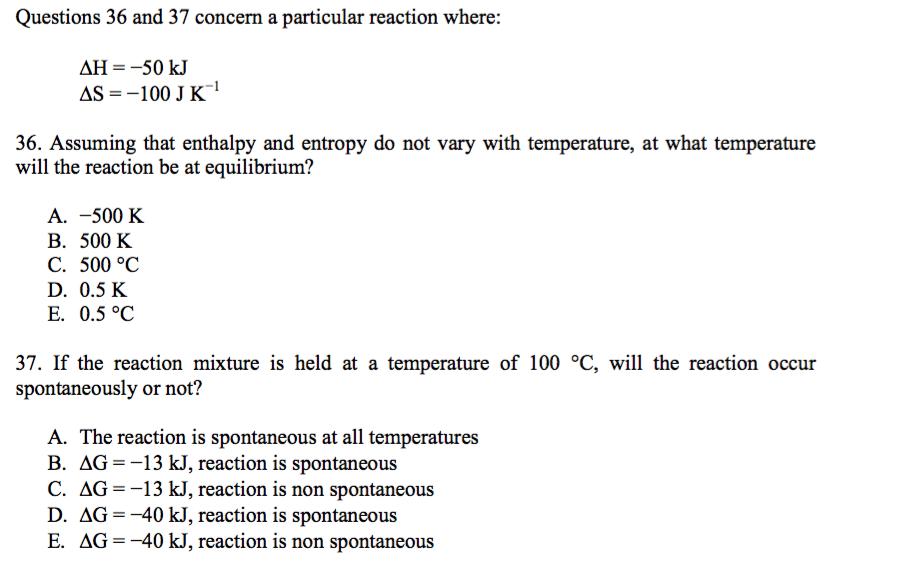
CHM135H1 Lecture Notes - Ammonia, Activation Energy, Methanol

90
CHM135H1 Full Course Notes
Verified Note
90 documents
Document Summary
Notes nothing to do with rate spontaneous reaction can be slow or fast for instance, diamond to graphite is a very slow yet spontaneous reaction if the forward reaction is spontaneous, the reverse is non- spontaneous. Delta h is not enough to determine spontaneity. S is a measure of disorder gas particles are very spread out. Entropy goes from localized to dispersion for spontaneous reactions. Whereas solid particles are in ordered structures. A) a2 (gas) + 2b (gas) -> 2ab (gas) B) negative s imagine two coins combinations of results are only hh; ht; th; tt therefore 2 to the power of 2 is four imagine three coins combinations of results can be hhh; ttt; hht; hth; tht; tth; More entropy in drop of water than a bucket because entropy is an extensive property. Delta s = s of cao plus s of co2 subtract s of caco3 = 1mol(39. 7j/kmol) + 1mol times 213. 6j/kmol 1 mol times.