CHM136H1 Lecture Notes - Lecture 11: Cyclohexane Conformation, Cyclohexane, Ring Strain
CHM136H1 verified notes
11/39View all
Document Summary
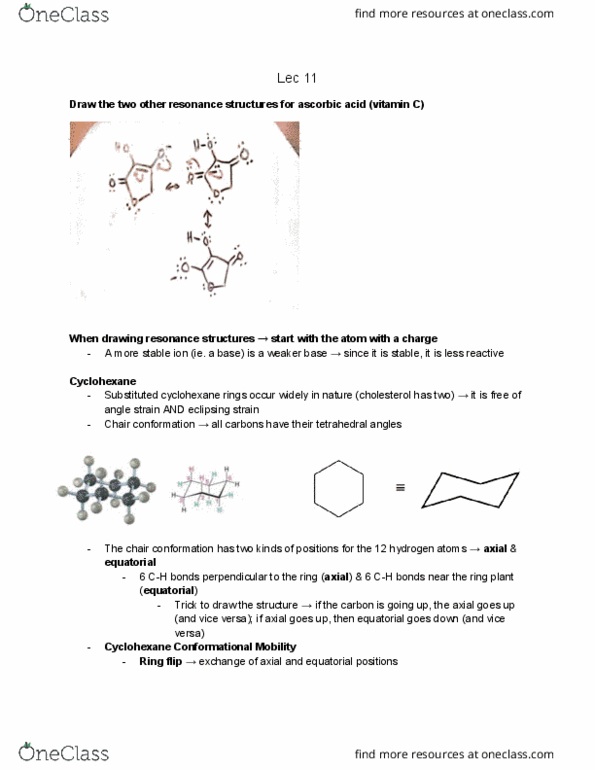
Draw the two other resonance structures for ascorbic acid (vitamin c) When drawing resonance structures start with the atom with a charge. A more stable ion (ie. a base) is a weaker base since it is stable, it is less reactive. Substituted cyclohexane rings occur widely in nature (cholesterol has two) it is free of angle strain and eclipsing strain. Chair conformation all carbons have their tetrahedral angles. The chair conformation has two kinds of positions for the 12 hydrogen atoms axial & equatorial. 6 c-h bonds perpendicular to the ring (axial) & 6 c-h bonds near the ring plant (equatorial) Trick to draw the structure if the carbon is going up, the axial goes up (and vice versa); if axial goes up, then equatorial goes down (and vice versa)