CHM136H1 Lecture Notes - Lecture 28: Halocarbon, Primary Alcohol, Bond Energy
CHM136H1 verified notes
28/39View all
Document Summary
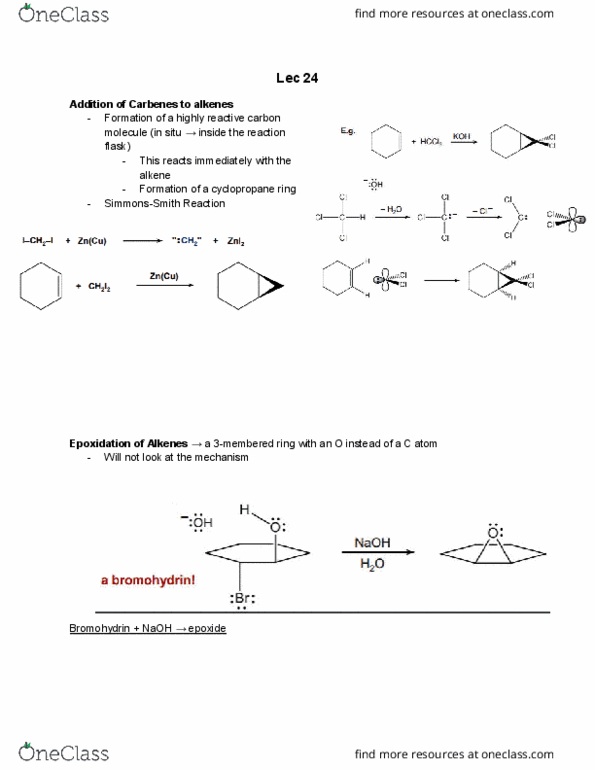
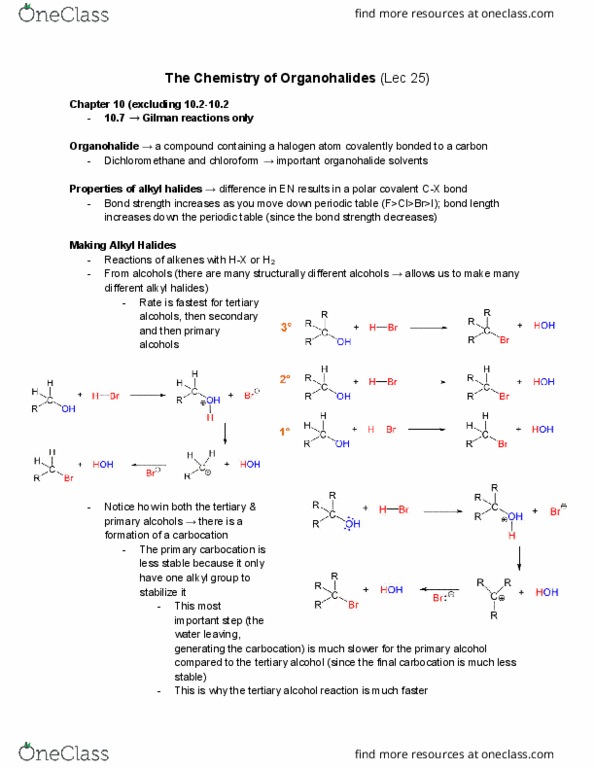
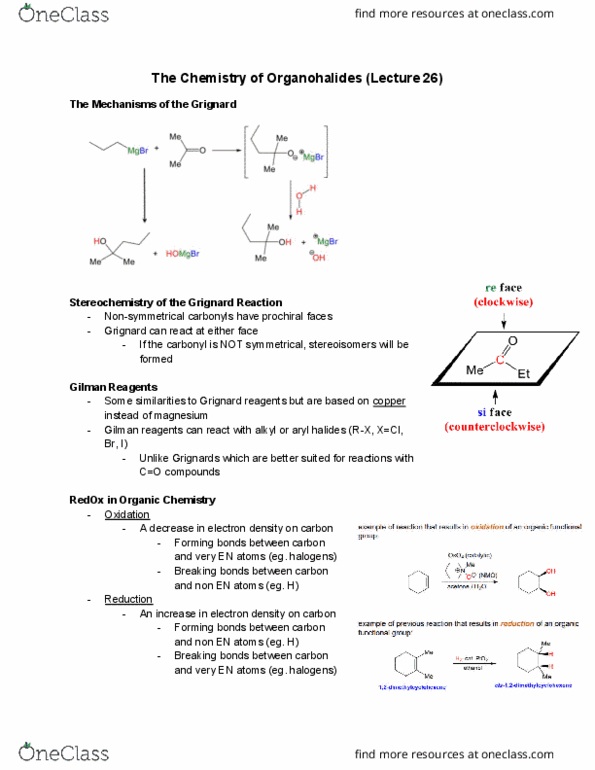
Organohalide a compound containing a halogen atom covalently bonded to a carbon. Dichloromethane and chloroform important organohalide solvents. Properties of alkyl halides difference in en results in a polar covalent c-x bond. Bond strength increases as you move down periodic table (f>cl>br>i); bond length increases down the periodic table (since the bond strength decreases) Reactions of alkenes with h-x or h2. From alcohols (there are many structurally different alcohols allows us to make many different alkyl halides) Rate is fastest for tertiary alcohols, then secondary and then primary alcohols. Notice how in both the tertiary & primary alcohols there is a formation of a carbocation. The primary carbocation is less stable because it only have one alkyl group to stabilize it. This most important step (the water leaving, generating the carbocation) is much slower for the primary alcohol compared to the tertiary alcohol (since the final carbocation is much less stable)