CHEM 101 Lecture Notes - Lecture 2: Electromagnetic Radiation, Sunburn, Transverse Wave
CHEM 101 verified notes
2/40View all
Document Summary
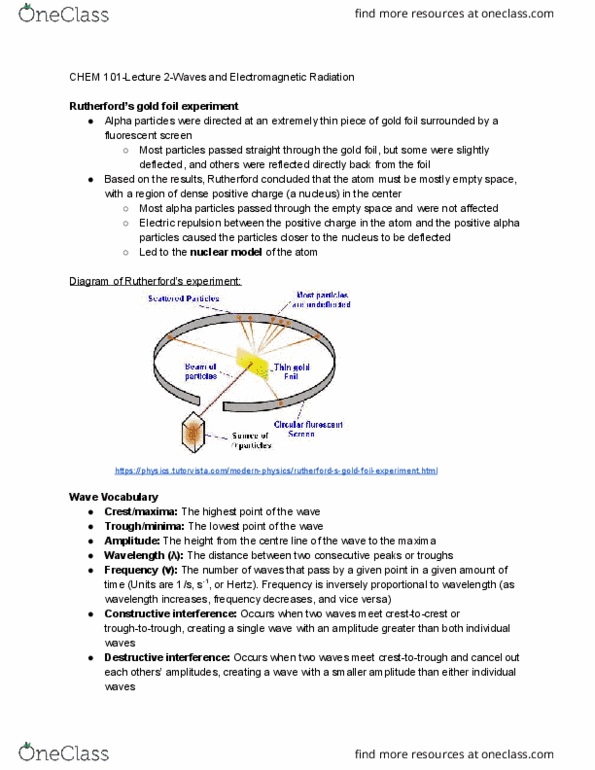
Alpha particles were directed at an extremely thin piece of gold foil surrounded by a fluorescent screen. Most particles passed straight through the gold foil, but some were slightly deflected, and others were reflected directly back from the foil. Based on the results, rutherford concluded that the atom must be mostly empty space, with a region of dense positive charge (a nucleus) in the center. Most alpha particles passed through the empty space and were not affected. Electric repulsion between the positive charge in the atom and the positive alpha particles caused the particles closer to the nucleus to be deflected. Led to the nuclear model of the atom. Crest/maxima: the highest point of the wave. Trough/minima: the lowest point of the wave. Amplitude: the height from the centre line of the wave to the maxima. Wavelength ( ): the distance between two consecutive peaks or troughs.