CHEM 101 Lecture Notes - Lecture 20: Lewis Structure, Lone Pair, Trigonal Bipyramidal Molecular Geometry
CHEM 101 verified notes
20/40View all
Document Summary
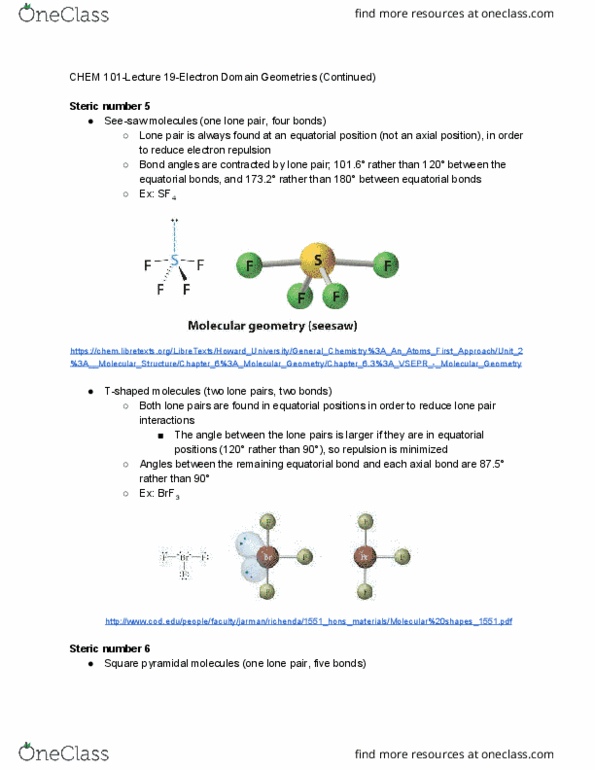
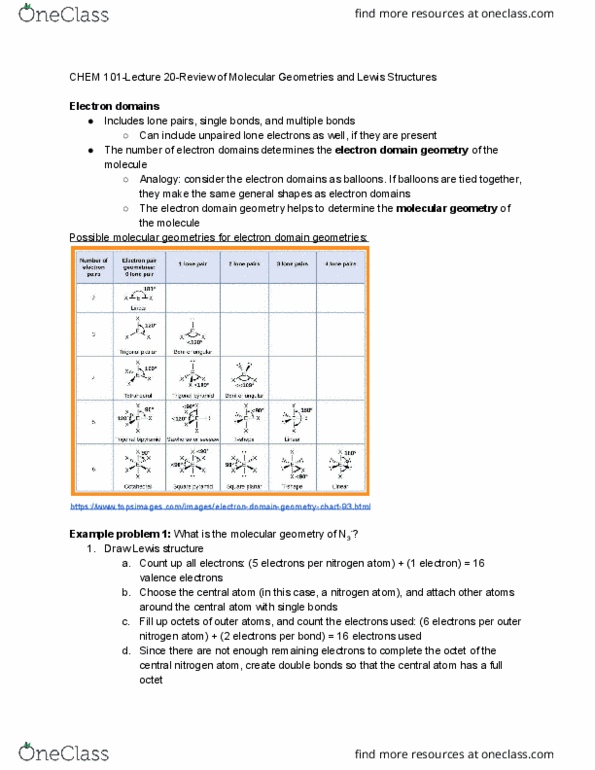
Chem 101-lecture 20-review of molecular geometries and lewis structures. Includes lone pairs, single bonds, and multiple bonds. Can include unpaired lone electrons as well, if they are present. The number of electron domains determines the electron domain geometry . Analogy: consider the electron domains as balloons. If balloons are tied together, they make the same general shapes as electron domains. The electron domain geometry helps to determine the molecular geometry . Possible molecular geometries for electron domain geometries: https://www. topsimages. com/images/electron-domain-geometry-chart-93. html. Finished lewis structure: https://www. quora. com/what-is-the-molecular-geometry-of-sef4-how-is-it-determined: determine electron domain geometry, count all electron domains around the central atom: (4 bonds) + (1 lone pair) = 5 electron domains, determine correct electron domain geometry: For five electron domains, the electron domain geometry is trigonal bipyramidal: determine molecular geometry, count the number of bonds and lone pairs: 4 bonds, 1 lone pair, use the electron domain geometry to.