Biochemistry 2280A Lecture Notes - Lecture 3: Lysine, N-Terminus, Alanine
Document Summary
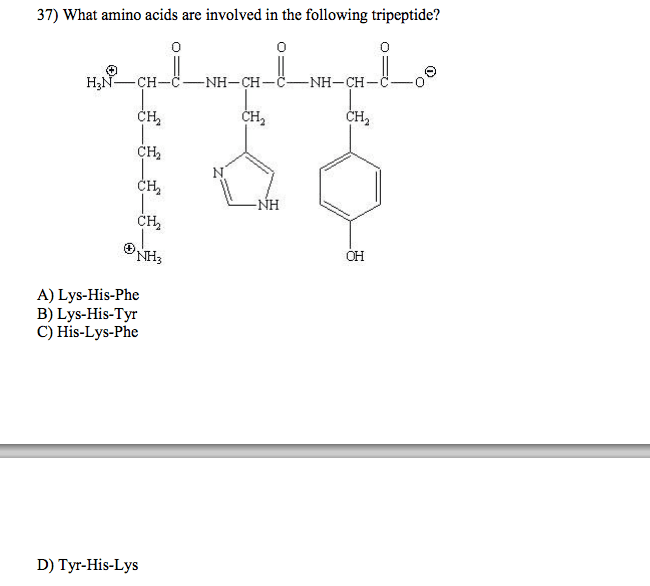
Not all proteins share 3d folding; some exist as natively unstructured proteins (random, unfolded conformations) The function of a protein depends on its structure which depends on the amino acid sequence. Are linear polymers of 50- 30,000 amino acids. The peptide bond is formed by the carboxyl group from one amino acid and the amino group (nh3+) of another. The reaction is a condensation reaction. There are polarities on the two opposite ends. Amino (n- ) terminal is positive; carboxyl (c- ) terminal is negative. Polypeptides are elongated through the c- terminal. The general pattern is n- c- c- n- c- c( aka the backbone) The peptide bond shares double bond characteristics through resonance between the c- o and c- n bonds. Bonds are all covalent. There is no rotation around the peptide bond; this constrains its flexibility and prevents certain folding patterns. Proteins structures have increasing complexity from primary secondary tertiary quaternary.