Engineering Science 1021A/B Lecture Notes - Lecture 24: Thermal Expansion, Glass, Boron Trioxide

Glasses have short range order
•
Silicates are glass-formers
As are B2O3, GeO2, P2O5, V2O3
○
If we cool these materials sufficiently quickly, they will not crystallize but
form glass
○
•
Silica Glasses
If molten silica is cooled relatively quickly, it is possible to prevent the formation
of a crystalline structure
•
Fused Silica is still made up of the Si
O4
tetrahedra, but not all oxygen atoms are
shared between two tetrahedra
Short-range but not long-range
○
•
•
Other oxides are added to change the properties of glass•
Network Formers fit in with the SiO2tetrahedra
Are added to change the properties of solidified glass
○
12% B2O3 is added to silica to make Pyrex. The addition lowers the
forming temperature without changing the thermal expansion coefficient
○
•
Network Modifiers do not fit in with the silica network
Make it easier to form a glass (as opposed to crystalline silica)
○
Too much modifier will segregate and cause crystalline silica to form
○
most glass contain up to 15% Na2O to make it easier to form a glass
○
Y2O3, MgO, CaO, PbO2, Na2O
○
•
Intermediates do not form glasses on their own, but fit into the silicate glass
structure and modify, for example, the fluidity or the glass transition
temperature
TiO2, ZnO, PbO, Al2O3, BeO
○
•
○
Workability of glass is viscosity dependent
○
•
Shows the effects of oxide additions and temperature on the viscosity of silicate
glasses
•
Fused silica
•
Soda-lime bottles
74%SiO2
○
15%Na2O – reduces softening temp.
○
5%CaO – as above
○
4%MgO – prevents crystallization
○
1%Al2O3– durability
○
•
Borosilicate – Pyrex
81% SiO2
○
4%NaO
○
2%Al2O3
○
12% B2O3– lowers softening temp without increasing expansion
coefficient
○
•
Processing Glasses
Deformation of non-crystalline ceramics is due to Viscous flow
•
Representation of viscous flow of a liquid or fluid glass in response to an
applied shear force
○
•
•
Viscosity is a measure of how difficult it is to shear a liquid
Water has high viscosity
○
Molasses has a high viscosity
○
•
When a crystalline material solidifies, there is a step change in volume at the
melting temperature
•
Glasses do not really solidify in the traditional sense
•
The molecules pack closer together, becoming an increasingly denser liquid
•
Slight change in slop occurs when the molecules are essentially unable to
flow
Glass Transition Temperature
§
○
•
•
Fabricating Glasses
Perfectly flat, parallel sided plate glass for windows are obtained by molten
glass is floated on top of molten tin (TM=231°C)
○
•
Tempered Glass
The fracture properties of glass can be altered by:
Laminating
○
tempering
○
•
Fracture patterns of glass in
Annealed statea)
Laminated stateb)
Tempered statec)
○
•
Temperatures and viscosities of glass in the tempering range
○
Room temperature residual stress distribution over the cross
section of a tempered glass
§
○
•
Tempering process and equipment:
Surface and midplane temperature profiles during heating and
cooling
i.
Heating portion of tempering furnaceii.
Quenching section of tempering furnaceiii.
○
•
Properties of Glass
Like crystalline ceramics:
Hard
○
Brittle
○
Corrosion resistant
○
•
Unlike crystalline ceramics:
Low melting temperatures
○
Can be easily deformed at high temperatures
○
Not porous
○
•
Glasses

Glasses have short range order•
Silicates are glass-formers
As are B2O3, GeO2, P2O5, V2O3
○
If we cool these materials sufficiently quickly, they will not crystallize but
form glass
○
•
Silica Glasses
If molten silica is cooled relatively quickly, it is possible to prevent the formation
of a crystalline structure
•
Fused Silica is still made up of the SiO4tetrahedra, but not all oxygen atoms are
shared between two tetrahedra
Short-range but not long-range
○
•
•
Other oxides are added to change the properties of glass•
Network Formers fit in with the SiO2tetrahedra
Are added to change the properties of solidified glass
○
12% B2O3 is added to silica to make Pyrex. The addition lowers the
forming temperature without changing the thermal expansion coefficient
○
•
Network Modifiers do not fit in with the silica network
Make it easier to form a glass (as opposed to crystalline silica)
○
Too much modifier will segregate and cause crystalline silica to form
○
most glass contain up to 15% Na2O to make it easier to form a glass
○
Y2O3, MgO, CaO, PbO2, Na2O
○
•
Intermediates do not form glasses on their own, but fit into the silicate glass
structure and modify, for example, the fluidity or the glass transition
temperature
TiO2, ZnO, PbO, Al2O3, BeO
○
•
○
Workability of glass is viscosity dependent
○
•
Shows the effects of oxide additions and temperature on the viscosity of silicate
glasses
•
Fused silica
•
Soda-lime bottles
74%SiO2
○
15%Na2O – reduces softening temp.
○
5%CaO – as above
○
4%MgO – prevents crystallization
○
1%Al2O3– durability
○
•
Borosilicate – Pyrex
81% SiO2
○
4%NaO
○
2%Al2O3
○
12% B2O3– lowers softening temp without increasing expansion
coefficient
○
•
Processing Glasses
Deformation of non-crystalline ceramics is due to Viscous flow
•
Representation of viscous flow of a liquid or fluid glass in response to an
applied shear force
○
•
•
Viscosity is a measure of how difficult it is to shear a liquid
Water has high viscosity
○
Molasses has a high viscosity
○
•
When a crystalline material solidifies, there is a step change in volume at the
melting temperature
•
Glasses do not really solidify in the traditional sense
•
The molecules pack closer together, becoming an increasingly denser liquid
•
Slight change in slop occurs when the molecules are essentially unable to
flow
Glass Transition Temperature
§
○
•
•
Fabricating Glasses
Perfectly flat, parallel sided plate glass for windows are obtained by molten
glass is floated on top of molten tin (TM=231°C)
○
•
Tempered Glass
The fracture properties of glass can be altered by:
Laminating
○
tempering
○
•
Fracture patterns of glass in
Annealed statea)
Laminated stateb)
Tempered statec)
○
•
Temperatures and viscosities of glass in the tempering range
○
Room temperature residual stress distribution over the cross
section of a tempered glass
§
○
•
Tempering process and equipment:
Surface and midplane temperature profiles during heating and
cooling
i.
Heating portion of tempering furnaceii.
Quenching section of tempering furnaceiii.
○
•
Properties of Glass
Like crystalline ceramics:
Hard
○
Brittle
○
Corrosion resistant
○
•
Unlike crystalline ceramics:
Low melting temperatures
○
Can be easily deformed at high temperatures
○
Not porous
○
•
Glasses
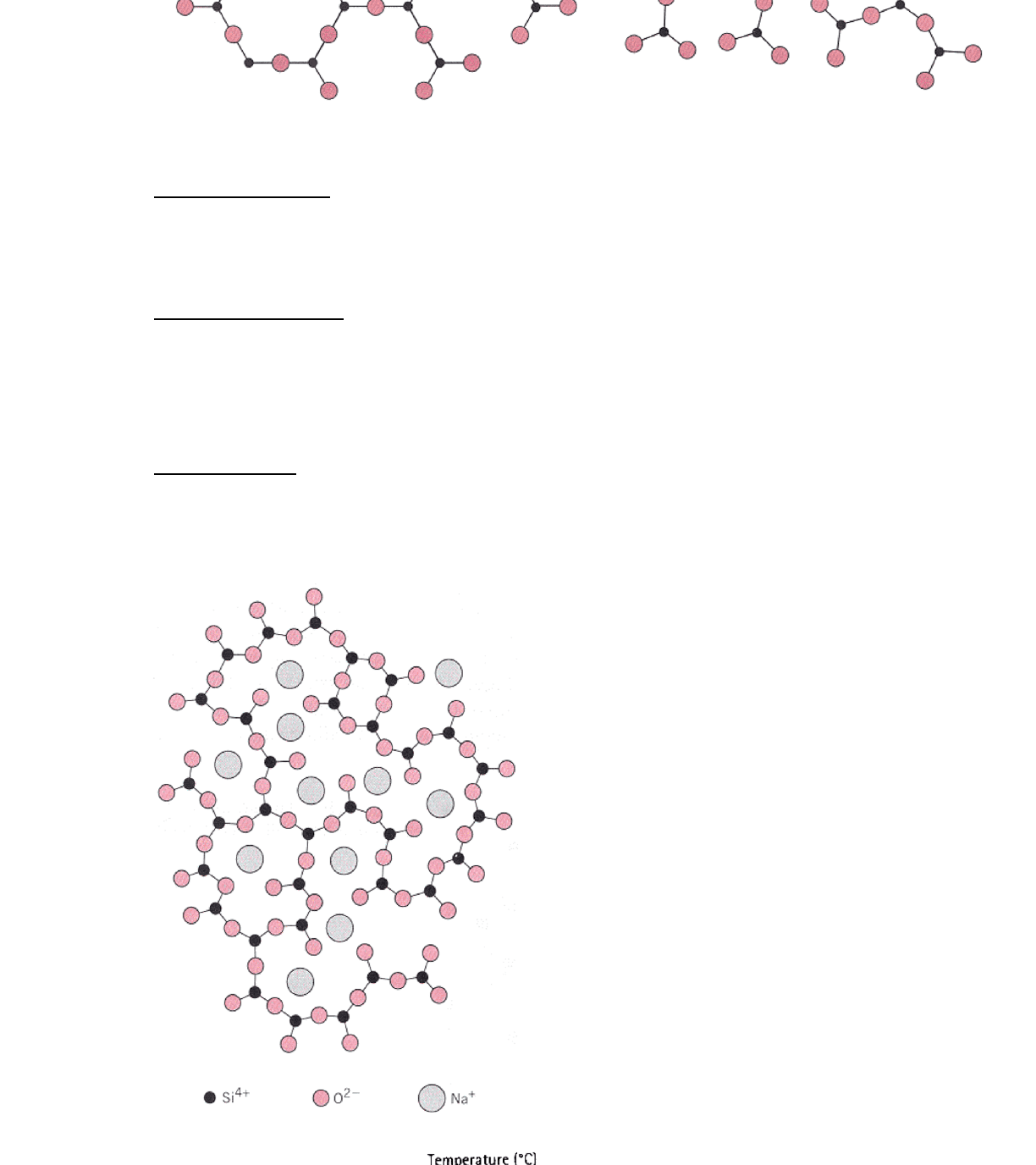
Glasses have short range order•
Silicates are glass-formers
As are B2O3, GeO2, P2O5, V2O3
○
If we cool these materials sufficiently quickly, they will not crystallize but
form glass
○
•
Silica Glasses
If molten silica is cooled relatively quickly, it is possible to prevent the formation
of a crystalline structure
•
Fused Silica is still made up of the SiO4tetrahedra, but not all oxygen atoms are
shared between two tetrahedra
Short-range but not long-range
○
•
•
Other oxides are added to change the properties of glass
•
Network Formers fit in with the Si
O2
tetrahedra
Are added to change the properties of solidified glass
○
12% B2O3 is added to silica to make Pyrex. The addition lowers the
forming temperature without changing the thermal expansion coefficient
○
•
Network Modifiers do not fit in with the silica network
Make it easier to form a glass (as opposed to crystalline silica)
○
Too much modifier will segregate and cause crystalline silica to form
○
most glass contain up to 15% Na2O to make it easier to form a glass
○
Y2O3, MgO, CaO, PbO2, Na2O
○
•
Intermediates do not form glasses on their own, but fit into the silicate glass
structure and modify, for example, the fluidity or the glass transition
temperature
TiO2, ZnO, PbO, Al2O3, BeO
○
•
○
Workability of glass is viscosity dependent
○
•
Shows the effects of oxide additions and temperature on the viscosity of silicate
glasses
•
Fused silica
•
Soda-lime bottles
74%SiO2
○
15%Na2O – reduces softening temp.
○
5%CaO – as above
○
4%MgO – prevents crystallization
○
1%Al2O3– durability
○
•
Borosilicate – Pyrex
81% SiO2
○
4%NaO
○
2%Al2O3
○
12% B2O3– lowers softening temp without increasing expansion
coefficient
○
•
Processing Glasses
Deformation of non-crystalline ceramics is due to Viscous flow
•
Representation of viscous flow of a liquid or fluid glass in response to an
applied shear force
○
•
•
Viscosity is a measure of how difficult it is to shear a liquid
Water has high viscosity
○
Molasses has a high viscosity
○
•
When a crystalline material solidifies, there is a step change in volume at the
melting temperature
•
Glasses do not really solidify in the traditional sense
•
The molecules pack closer together, becoming an increasingly denser liquid
•
Slight change in slop occurs when the molecules are essentially unable to
flow
Glass Transition Temperature
§
○
•
•
Fabricating Glasses
Perfectly flat, parallel sided plate glass for windows are obtained by molten
glass is floated on top of molten tin (TM=231°C)
○
•
Tempered Glass
The fracture properties of glass can be altered by:
Laminating
○
tempering
○
•
Fracture patterns of glass in
Annealed statea)
Laminated stateb)
Tempered statec)
○
•
Temperatures and viscosities of glass in the tempering range
○
Room temperature residual stress distribution over the cross
section of a tempered glass
§
○
•
Tempering process and equipment:
Surface and midplane temperature profiles during heating and
cooling
i.
Heating portion of tempering furnaceii.
Quenching section of tempering furnaceiii.
○
•
Properties of Glass
Like crystalline ceramics:
Hard
○
Brittle
○
Corrosion resistant
○
•
Unlike crystalline ceramics:
Low melting temperatures
○
Can be easily deformed at high temperatures
○
Not porous
○
•
Glasses
Document Summary
If we cool these materials sufficiently quickly, they will not crystallize but form glass. If molten silica is cooled relatively quickly, it is possible to prevent the formation of a crystalline structure. Fused silica is still made up of the sio4 tetrahedra, but not all oxygen atoms are shared between two tetrahedra. Other oxides are added to change the properties of glass. Network formers fit in with the sio2 tetrahedra. Are added to change the properties of solidified glass. 12% b2o3 is added to silica to make pyrex. The addition lowers the forming temperature without changing the thermal expansion coefficient. Network modifiers do not fit in with the silica network. Make it easier to form a glass (as opposed to crystalline silica) Too much modifier will segregate and cause crystalline silica to form most glass contain up to 15% na2o to make it easier to form a glass.

