CHE 350 Lecture Notes - Lecture 2: Protein Structure, Oligomer, Cyclic Group
Chapter 6 – Protein Structure
1) Primary = linear sequence of amino acids (primary structure dictates 3D structure)
2) Secondary = local spatial arrangement of a polypep’s backbone atoms w/o regard to R-
group conformation (ALL H-BONDS LOCALIZED)
3) Tertiary = 3D structure of an entire polypeptide, including its R-groups (H-BONDS FROM
FAR AWAY + AMINO ACID RESIDUES GOING IN, OUT, OR ON PROTEIN)
4) Quaternary = spatial arrangement of the subunits (proteins w/ ≥2 polypep chains)
Secondary Structure (Helices, Sheets, Turns)
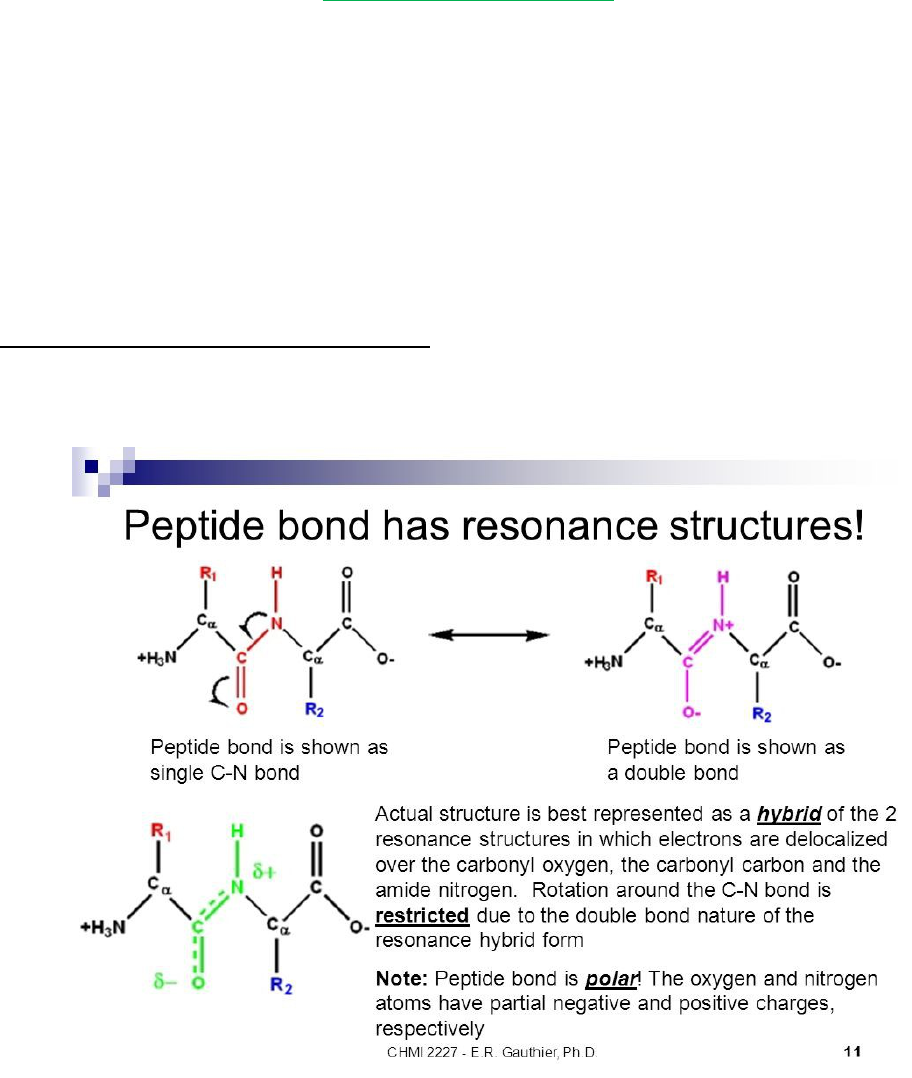
Peptide group rigid, planar structure as a consequence of resonance interxns that give
peptide bonds ~40% double-bond character
Peptide group’s C-N bond is shorter than the N-Cα single bond and the C=O bond is
longer than that of aldehyde’s and ketones
oPlanar conformation maxes π-bonding overlap, giving it rigidity
find more resources at oneclass.com
find more resources at oneclass.com
Usually peptide groups are TRANS
Cis conformation is less stable due to steric hindrance w/ side chains
oThis is reduced in peptide bonds to Pro residues, so ~10% of Pro residues are cis
peptide bonds
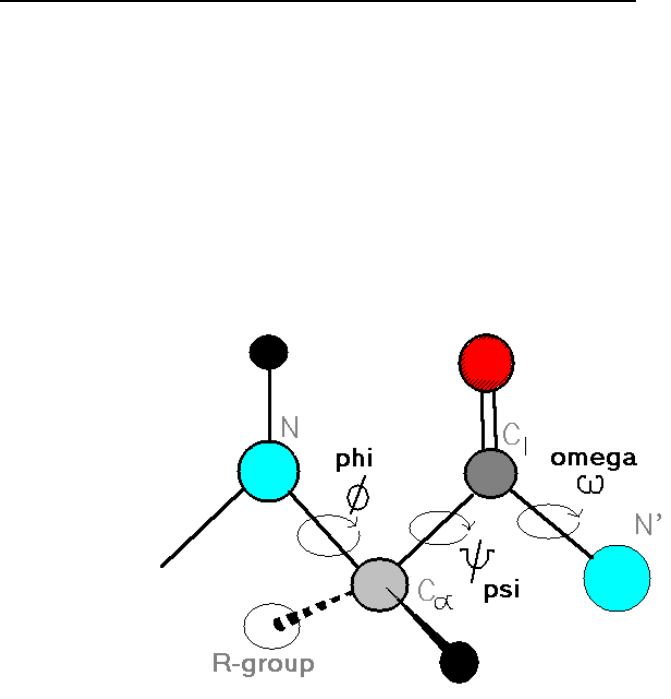
Torsion Angles b/w Peptide Groups Describe Chain Conformation
Backbone = main chain of protein = atoms that participate in peptide bonds (ignoring
side chains)
oDrawn as linear links of rigid, planar peptide groups
Conformation of backbone can be described by TORSION ANGLES (dihedral angles,
rotation angles) around the Cα –N bond (φ) and Cα –C bond (ψ) of each amino acid
oAngles are 180⁰ when polypeptide chain is in its fully extended conformation
oIncreases clockwise when viewed from Cα
TORSION ANGLES (conformational freedom) of polypeptide backbones are STERICALLY
STRAINED
oEverything can collide
oThings closer than their van der Waals distance are sterically forbidden
oVan der Waals = distance of closest contact b/w non-bonded atoms
Proline (Pro) is the most conformationally restricted amino acid residue = cyclic side
chain BULKY
Glycine (Gly) has no CB atom much less sterically hindered (assumes conformations
forbidden to other residues)
find more resources at oneclass.com
find more resources at oneclass.com

Most Common Secondary Structures = ALPHA HELIX ( α -HELIX), BETA SHEET ( β -SHEET)
Repeating φ and ψ values
1) ALPHA HELIX (α-HELIX)
a. Both favorable H-bonding pattern and torsion angle values (φ/ψ) that are fully
allowed
b. Arrangement
b.i. Backbone H-bonds arranged such that the peptide C=O bond of the nth
residue points along the helix axis toward the peptide N-H group of the (n
+ 4)th residue = strong H bonds with optimal distance of N- - - O
b.ii. Amino R groups project out and down from helix, avoiding steric
interference w/ backbone and each other
b.iii. Core of helix is tightly packed (atoms are in van der Waals contact)
2) BETA SHEET (β-SHEET)
find more resources at oneclass.com
find more resources at oneclass.com
Document Summary
Far away + amino acid residues going in, out, or on protein: quaternary = spatial arrangement of the subunits (proteins w/ 2 polypep chains) Peptide group rigid, planar structure as a consequence of resonance interxns that give peptide bonds ~40% double-bond character. Peptide group"s c-n bond is shorter than the n-c single bond and the c=o bond is longer than that of aldehyde"s and ketones: planar conformation maxes -bonding overlap, giving it rigidity. Cis conformation is less stable due to steric hindrance w/ side chains: this is reduced in peptide bonds to pro residues, so ~10% of pro residues are cis peptide bonds. Torsion angles b/w peptide groups describe chain conformation. Backbone = main chain of protein = atoms that participate in peptide bonds (ignoring side chains: drawn as linear links of rigid, planar peptide groups. Torsion angles (conformational freedom) of polypeptide backbones are sterically.

