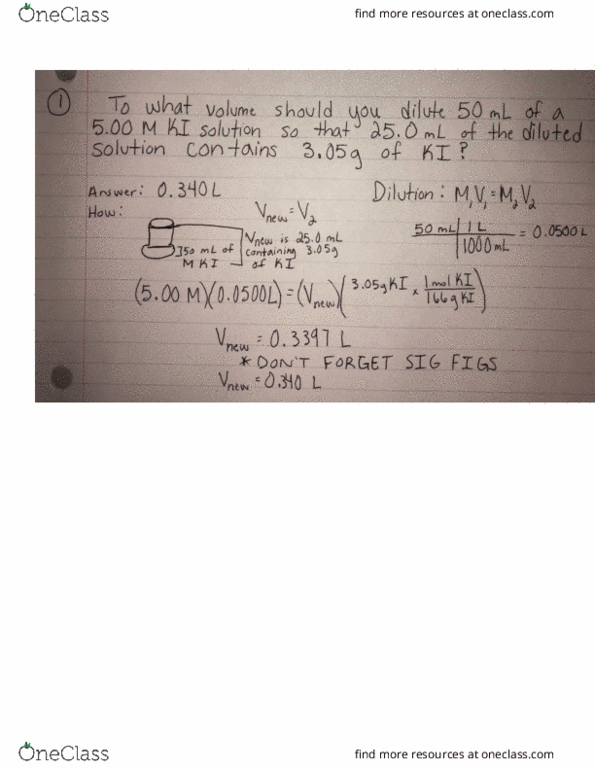CHM 111 Lecture Notes - Lecture 24: Dimensionless Quantity, Equilibrium Constant, Exothermic Process
CHM 111 verified notes
24/31View all
Document Summary
Chapter 15: chemical equilibrium (review) and practice problems. Reactions where the reactant and product are in different phases. Finding equilibrium concentrations when given the equilibrium constant, initial concentrations, or pressure: determine the direction of the reaction using the comparison of qc and kc, determine the changes of the materials (in terms of x, solve for x. Approximations to simplify the math: a small equilibrium constant will favor the reactants, to approximate the equilibrium concentration: xequilibrium xinitial when assuming that the reaction is a forward reaction. Le ch telier"s principle: the position of an equilibrium will shift when the system is being disturbed. It will move to a position of less disturbance: concentration, shifts the equilibrium to the left: Decrease in concentration of the reactants: shift the equilibrium to the right: Decrease in concentration of the products: volume: Increase = moves to the side with the most moles of gas.