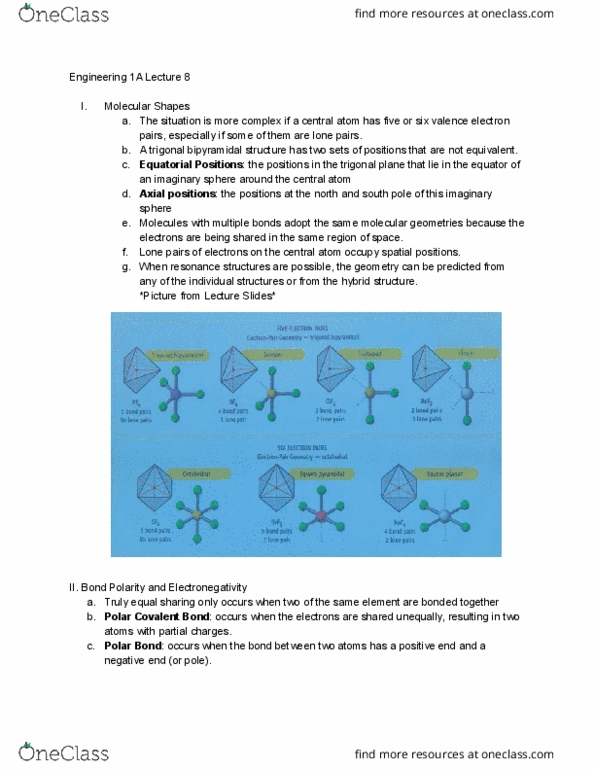
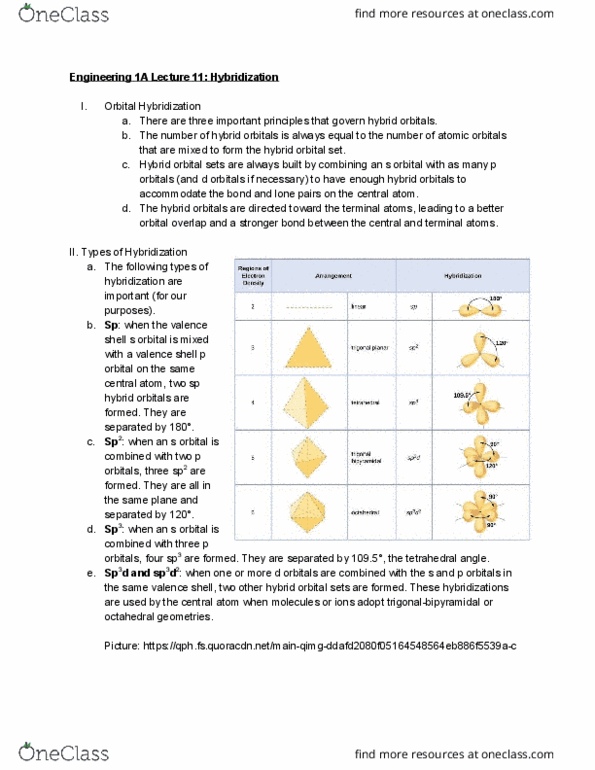
ENGR 1A Lecture Notes - Lecture 9: Valence Bond Theory, Linus Pauling, Covalent Bond
ENGR 1A verified notes
9/31View all
Document Summary
R= (number of share pairs in all x-y bonds)/(number of x-y links in the. Bond properties: bond orde , bond dissociation enthalpy , related to the order of the bond, always a positive quantity because energy is absorbed e, allows us to calculate the net change in energy when bonds are broken and. : the enthalpy change for breaking a bond in a molecule with the reactants and products in the gas phase indicates the relative stability of molecules formed in a chemical reaction. Molecule(g) energy supplied = h >0 molecular fragments (g) : bonding between two atoms occurs when the electron clouds on two atoms interpenetrate or overlap. This increases the probability of finding bonding electrons in the region of space between the two nuclei: the idea that bonds are formed by overlap of atomic orbitals is the basis for the valence bond theory . : the covalent bond that arises from the overlap of two s orbitals.