CHM 2046 Lecture Notes - Lecture 2: Partial Pressure, Molar Concentration, Kilogram
Document Summary
Get access


Related Documents
Related Questions
Seperation lab: Will rate question
Separation lab - will choose best answer
Seperation lab: will choose best answer
Separation of a Mixture Laboratory
In this experiment we will separate the different components of a mixture made up of sand (SiO2) and sodium chloride (NaCl, commonly known as salt). Both of these compounds are solid white crystals, so it is difficult to tell them apart with the naked eye. However, each component exhibits different physical properties that can be used to separate one from the other. There are several physical separation techniques that can be used to determine the percent composition of the original mixture.
Materials & Apparatus:
Sand/salt mixture provided in your Lab Kit
2 - Erlenmeyer Flasks
Scale
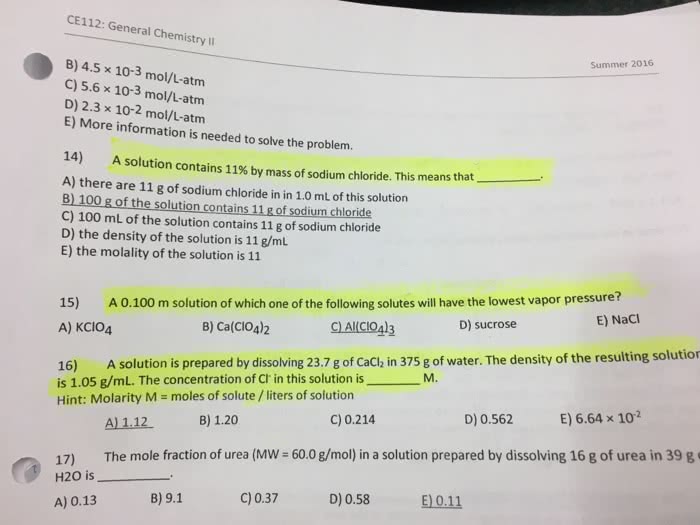
Water
Graduated Cylinder
Background:
It is important to know how to separate mixtures because many reactions carried out in the laboratory produce mixtures of different compounds. It is often necessary to separate the components of these mixtures, which can be done using several techniques. We will explore a few of these techniques: sublimation, filtration, evaporation and decantation.
Sublimation is the process of a substance passing from the solid to the gaseous state without first passing through the liquid state. Not all substances possess this characteristic. In fact, most substances to NOT sublime under normal conditions, but must be placed under low pressure and low temperature. Ammonium chloride (NH4Cl) and iodine (I2) are both substances that do sublime under normal conditions.
Filtration is the process used to separate the components of a mixture of solid substances that differ in solubility in a certain solvent. You can separate a mixture of solid substances by adding a liquid to the original mixture in which only some of the components of the mixture will dissolve. The resulting mixture, which contains both the dissolved and undissolved substances, is then poured through a filter paper--a porous paper that allows liquids to pass through while retaining any solids. The liquid that has passed through the filter paper is called the filtrate; the solid on the paper is the residue.
Evaporation can also be used to separate the solvent from the solution. A solution can be slowly heated in an Erlenmeyer flask, and the solute will remain after the solvent has evaporated.
Decantation is the process of separating a liquid from a solid (sediment) by gently pouring the liquid from the solid so as not to disturb the solid.
For this experiment, a practical plan is as follows: First combine the solid mixture of the two compounds, sodium chloride (NaCl) and sand (SiO2), with water. This will dissolve the sodium chloride. The sand will not dissolve into the water. We will "filter" the entire mixture, which allows the water in which the sodium chloride is dissolved to pass through, but not the sand. We will now have separated the sodium chloride and the sand. Then, we must evaporate off the water so that we weigh only the two original solids and not the water we added.
Procedure:
(NOTE: Do NOT dispose of any part of your lab until you are 100% positive you do not need it again. It is good practice to keep all parts of your lab until you are finished.)
Record the letter (A, B, C, or D) of your mixture (on the bag contents label) on the line above Data Table 1 below.
Obtain an Erlenmeyer flask [label it 1] and weigh and record the mass.
Add the entire contents of your unknown mixture bag to the flask and weigh and record the mass of the Erlenmeyer flask and the mixture.
Add 10 mL of water (preferably distilled) to the solid in the Erlenmeyer flask [Erlenmeyer flask 1] and stir gently for 5 minutes.
Weigh a new clean, dry Erlenmeyer flask [Erlenmeyer flask 2].
Decant the liquid from Erlenmeyer flask 1 into Erlenmeyer flask 2. This may work better if you place a utensil against the lip of the pouring flask to encourage a slow steady stream of liquid into the second flask.
Add another 10 mL of water to the solid in Erlenmeyer flask 1, stir for 1-2 minutes, and decant this liquid into the Erlenmeyer flask 2 as before.
Repeat with still another 10 mL of water. This process extracts (dissolves) the soluble sodium chloride from the insoluble sand.
Allow the two flasks to dry. Let them dry until the sand appears dry and freely moves when you shake the Erlenmeyer flask a little and the sodium chloride looks dry.
To speed up the process, you can heat the flasks slowly over a burner set on low on your stove top or in the oven (set at 125-200ËF). I would suggest heating it on top of another pan (NOT nonstick coated) Use a low setting and watch closely as you donât want it to boil over.
Allow the Erlenmeyer flasks to cool to room temperature (if they were heated) and weigh the Erlenmeyer flasks and their contents separately. Record their masses in the appropriate sections of the table.
To ensure that there is no water remaining, heat for another 5-10 minutes oven or 1-2 minutes on the stove. Allow to cool to room temperature and reweigh the Erlenmeyer flask. If it is within 0.3 g of your initial weighing you can stop. If the second weighing is not within 0.3 g of your initial weighing, reheat again, cool, and reweigh. The difference between this mass and the mass of the empty Erlenmeyer flask is the mass of NaCl.
Unknown Mixture Label (A, B, C, or D) ___________
Data Table 1
| Measure or calculate and record the following (in grams): | |
| mass of the empty Erlenmeyer flask 1: | |
| mass of the Erlenmeyer flask 1 plus the mixture sample: | |
| mass of the mixture sample: | |
| mass of the Erlenmeyer flask 1 and the remaining contents after drying of sand: | |
| mass of Erlenmeyer flask 1 and dry sand (2nd weighing) | |
| Subtract (a) from (e) to obtain the mass of sand in the sample: | |
| Measure or calculate and record the following (in grams): | |
| mass of an empty Erlenmeyer flask 2: | |
| mass of Erlenmeyer flask 2 and dry NaCl (1st weighing) | |
| mass of Erlenmeyer flask 2 and dry NaCl (2nd weighing) | |
| mass of Erlenmeyer flask 2 and dry NaCl (3rd weighing) if necessary | |
| measured mass of dry NaCl: |
Assignment Questions (all work must be shown for all calculations):
Using the results of your lab, complete all the questions below.
Upload one picture that is representative of your experiment with this report to the appropriate D2L Brightspace Assignments folder.
Complete Data Table 1 located in the Procedure. Make sure to include all your work for all the calculations performed.
The amount of sodium chloride in the original mixture may also be determined indirectly by subtracting the SiO2 mass from the initial sample mass. Calculate the expected mass of NaCl using this method.
Calculate the percent difference of NaCl (Note: This is not % Error, but it is a similar calculation. This is an error calculation that checks precision between the results of the two methods from the following equation: (Note: the unit for this calculation is â% differenceâ)
% difference=measured mass-mass by subtractionmass by subtraction x 100
What is the mass percent of each component in your mixture: SiO2, and NaCl? (Note: the units for this calculation are â% SiO2â and â% NaClâ)
mass percent=mass of componenttotal mass of sample x 100
Error Analysis: Was your % difference from Assignment Question 4 within 10%? Why or why not? Did your two mass percentages in Assignment Question 5 add up to 100%? Why or why not? What were your biggest sources of error in this experiment? What would you do differently if you were to repeat this experiment? Explain.
What type of properties (physical or chemical) are we using to separate the mixture in this laboratory. Explain your answer.
A mixture is found to contain 0.69 g SiO2, 1.05 g of cellulose, and 2.17 g of calcium carbonate. What is the mass percentage of SiO2 in this mixture?
Assume you have a mixture of NH4Cl, sand, and salt and access to a regular chemistry laboratory. How would you separate the three?Write out what your procedure would be in numbered steps. Please research the answer to this question and properly cite your source. It is alright to research using the internet. However, Wikipedia, Yahoo Answers, Ask.com, and other non-scientific sources cannot be used.