CH ENGR 104C Lecture Notes - Lecture 21: Lewis Structure, Pi Bond, Covalent Bond
58 views3 pages
7 Feb 2017
School
Department
Course
Professor
Document Summary
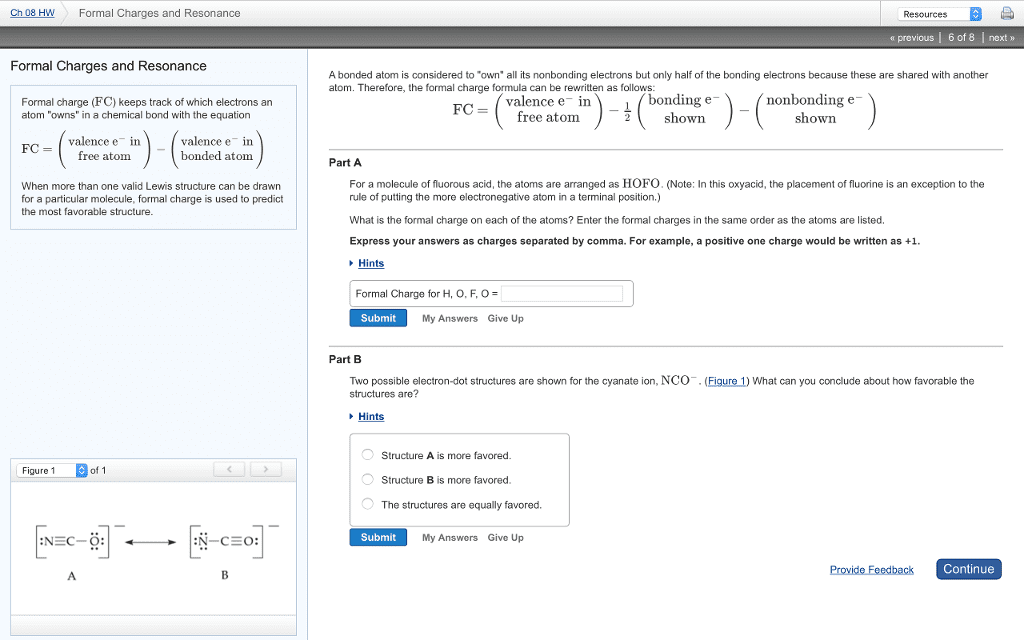
There are many occasions in which two or more valid lewis structures are used to illustrate a compound"s structure. Even though these lewis structures differ in the placement of their electrons, they all represent the same compound. It is important to understand that none of these lewis structures has a real physical existence, and the best representative of reality is the weighted average of these lewis structures. Resonance contributor: each lewis structure that represents a compound"s real structure to a degree. Since these structures do not have a real physical existence it is wrong to assume that they are in equilibrium with each other. Example 1: these are two valid lewis structures for ch3och2. *the above structures are not in equilibrium with each other; therefore, the resonance sign used to illustrate their relationship with each other. Resonance hybrid: the weighted average (blend) of resonance contributors which best represents the reality. Demonstrating the molecule the way it is observed in lab.
Get access
Grade+20% off
$8 USD/m$10 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
40 Verified Answers
Class+
$8 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
30 Verified Answers