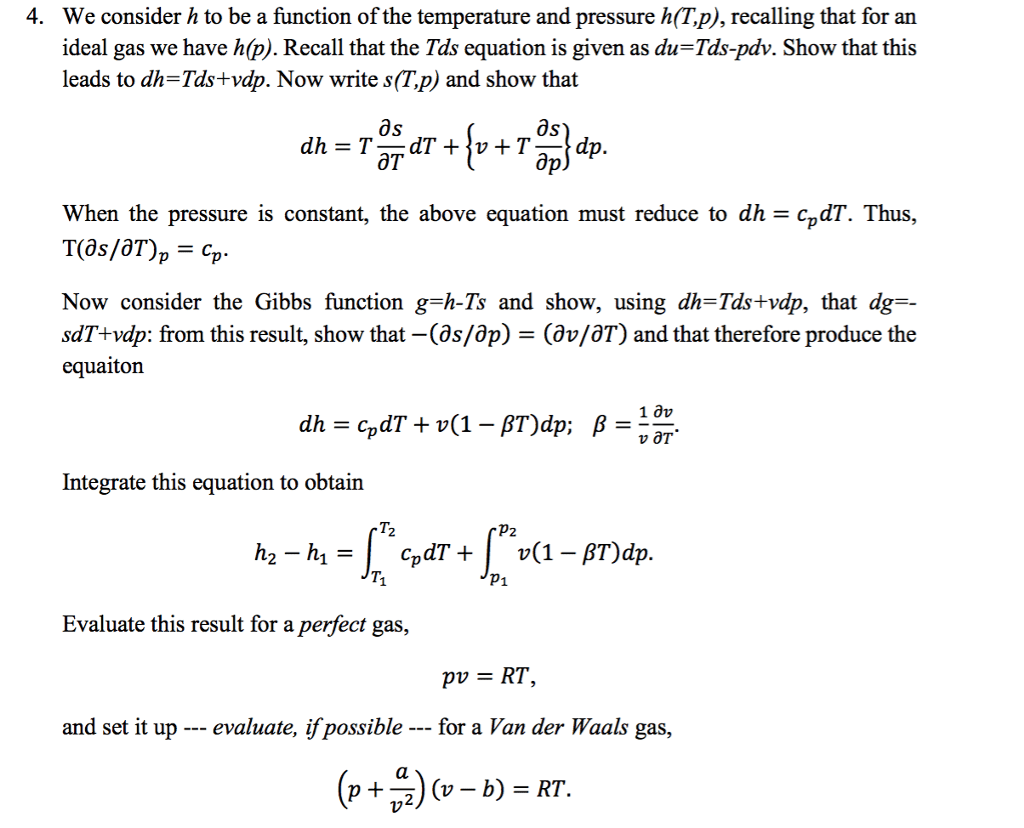CENG 102 Lecture Notes - Lecture 3: Real Gas, R V R, Ideal Gas
Document Summary
Need to use eos information we learned and apply to accurately determining h, u, g, a, s. Turns out g can be used to calculate everything else dg = vdp sdt. P & t are easy to measure, so we can easily determine g(p,t) Therefore, from g/rt, we can determine v, h, s, & u. We ultimately want to measure thermodynamic properties of real substances. We know how to do this for ideal gases. Ideally, we would be able to calculate for an ideal gas and then add a correction factor to get the real gas property. Introduce residual properties: mr the difference between real and ideal gas properties at a given t, p. Bottom line: we can do calculations with an ideal gas, and simply add a correction term. We must determine relationships to evaluate residual properties. Vr = v vig = v . Thus, we can evaluate vr, gr, hr, and sr at constant t.