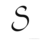3
answers
0
watching
462
views
13 Dec 2019
calculate the Solubility of Ag2CrO4 (expressed as moles ofCrO4^2- per liter) in
a) 0.050M KClO4
b) 0.0050M AgNO3
I have no idea how to start this question.
calculate the Solubility of Ag2CrO4 (expressed as moles ofCrO4^2- per liter) in
a) 0.050M KClO4
b) 0.0050M AgNO3
I have no idea how to start this question.
20 Dec 2021
Already have an account? Log in