Biochemistry 2280A Lecture Notes - Lecture 10: Lipoprotein, High-Density Lipoprotein, Glycerophospholipid
22 views2 pages
25 Aug 2013
School
Department
Course
Professor
Document Summary
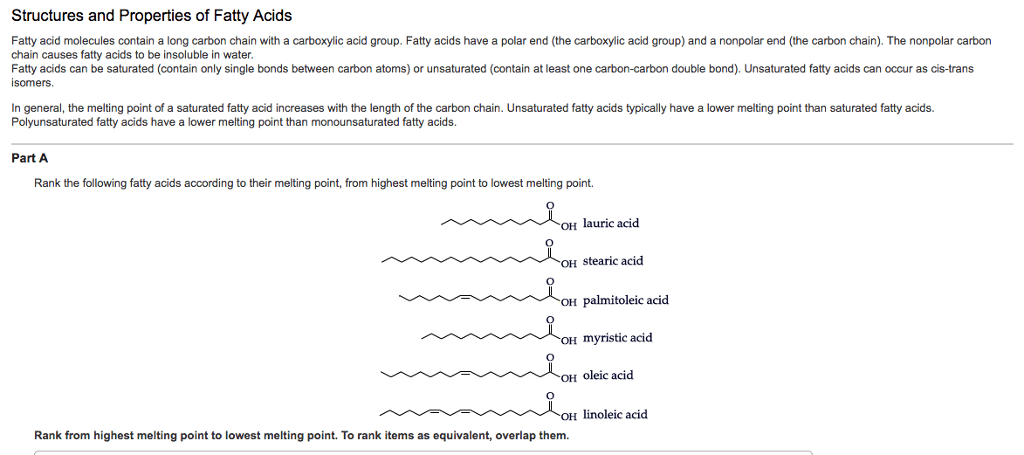
A lipid is a biological molecule that is not very soluble in water (practically insoluble means hydrophobic). An example of a practical application of lipids in the body is the lipid bi-layer of which all cell membranes are comprised. Saturated fatty acids are saturated with hydrogen atoms. Monounsaturated fatty acids contain 1 element of unsaturation (1 double bond). there are 2 ways you can make unsaturated fats: as a trans or cis stereoisomer. The difference between the two is in the orientation of the substituents (hydrogen atoms) as there can be no rotation about the double bond. The cis stereoisomer causes fatty acids to form a kink (bend in its shape). Double bonds in biological systems will tend to put bonds in specific places (between carbons 9 and 10). Cis double bond arrangements are the norm; trans double bond arrangements are rare in nature (synthetic).
Get access
Grade+20% off
$8 USD/m$10 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
40 Verified Answers
Class+
$8 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
30 Verified Answers