CHEM 1151 Study Guide - Fall 2018, Comprehensive Midterm Notes - Unified Atomic Mass Unit, Relative Atomic Mass, Chemical Equilibrium

CHEM 1151
MIDTERM EXAM
STUDY GUIDE
Fall 2018
Unit 1 Notes
1. Chemistry: the study of matter and the interactions that it undergoes
2. Matter: anything that has mass and volume
a. States of matter
i. Solid: definite shape, definite volume
ii. Liquid: no definite shape, definite volume
iii. Gas: no definite shape, no definite volume
b. Properties of matter
i. Physical properties: properties that give characteristics of a material
without changing the material (boiling point (BP), melting point (MP),
color, density, hardness, etc.)
ii. Chemical properties: properties that describe how a material will interact
with another material
c. Changes in matter
i. Physical changes: changes that do not involve a change in the
composition of a substance (i.e. change of state, ripping, tearing, etc.)
ii. Chemical changes: changes that involve a change in the composition of
the substance (i.e. rusting, cooking, combustion, etc.)
3. Substance: anything that is pure
4. Element: a pure substance that cannot be simplified or broken down into simpler things
by means of a chemical reaction
5. Compound: two or more elements that have chemically combined
6. Mixture: 2 or more substances that are not chemically combined
a. Homogeneous: looks the same throughout
b. Heterogeneous: looks different throughout
7. Measurements: a good measurement will have both a number and a unit. In science,
the metric unit is the preferred system
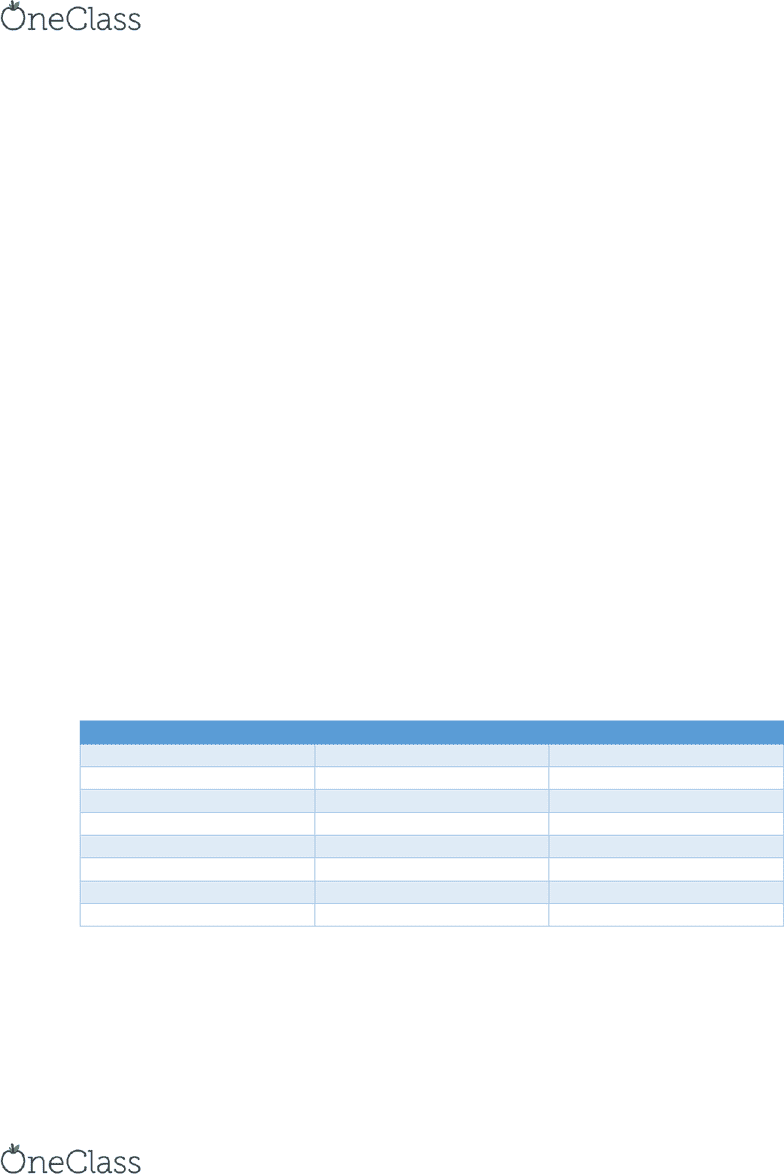
a. Metric conversions: M _ _ k h D b d c m _ _ µ
Prefix
Abbreviation
Meaning
Mega
M
1 E 6 (1,000,000)
Kilo
k
1 E 3 (1,000)
Hecta
h
1 E 2 (100)
Deca
D
1 E 1 (10)
Deci
d
1 E -1 (0.1)
Centi
c
1 E -2 (0.01)
Milli
m
1 E -3 (0.001)
Micro
µ
1 E -6 (0.000001)
8. Mass: the amount of matter (stuff) in an object
9. Volume: the amount of space an object takes up
10. Length: distance between two points
11. Scientific notation: a uer that’s ultiplied y to soe power
find more resources at oneclass.com
find more resources at oneclass.com
a. Standard format—number can only have one nonzero digit to the left of the
decimal
b. Positive exponent means a number > 1, negative exponent means a number < 1
c. Examples: 1.25 E-2 = 0.0125, 1.25 E2 = 125
12. Significant figures (sig figs)
a. Atlantic/Pacific rule: if a decimal point is absent, count from the Atlantic (right)
side of the number starting with the first nonzero digit. If a decimal point is
present, count from the Pacific (left) side of the number starting with the first
nonzero digit.
b. Defined numbers or counting numb ers have an infinite number of sig figs
i. Defined number: determined by dictionary definition (i.e. 1 minute = 60
seconds)
ii. Coutig uers: you a touh while you’re outig
13. Rounding off numbers: 0-4, drop; 5-9, round up
14. Add/subtract: the answer will have he same number of digits fo the right of the decimal
as the measurement that has the fewest digits to the right of the decimal
15. Multiply/divide: the answer will have the same number of sig figs as the measurement
with the fewest number of sig figs
16. Unit analysis: method of changing from one set of units to another by using a series of
conversion factors
a. Conversion factor: fraction made from an equivalency (i.e. 1 ft = 12 in)
b. Conversion units are set up so that the same units are on the diagonal
c. Example: how many minutes are in 4.57 weeks?
17. Density
a. Density: ratio of mass to volume of an object
b. Units are usually g/ml, but they can be any mass/any volume
c. Any pure substance can be identified by its density
d.
e. Density of pure water is 1.000 g/ml
i. Anything with a larger density will sink, anything with a smaller density
will float
18. Temperature
a. Temperature: measure of the average kinetic energy of a substance
i. Kinetic energy: how fast particles move
b. Celsius—°C = K – 273
c. Kelvin—K = °C + 273
d. Water freezing point: 0 °C = 273 K = 32 °F
e. Body temperature: 37°C = 310. K = 98.6 °F
f. Water boiling point: 100 °C = 373 K = 212 °F
g. Absolute zero: -273 °C = 0 K = -459 °F
h. Liquid nitrogen boiling point: -196 °C = 77 K = -321 °F
find more resources at oneclass.com
find more resources at oneclass.com
Document Summary
Fall 2018: chemistry: the study of matter and the interactions that it undergoes, matter: anything that has mass and volume. In science, the metric unit is the preferred system: metric conversions: m _ _ k h d b d c m _ _ . Gain/lose e- lose 1 lose 2 lose 3 tweener gain 3 gain 2 gain 1 neither. 0: writing formulas of ionic compounds, write positive ion followed by negative ion, cross charges to give subscripts. If more than 1 polyatomic ion is needed, use parenthesis around the polyatomic ion, then use the subscript: reduce the subscripts (outside of the parenthesis) to lowest whole number, polyatomic ions. Lead (ii or iii: naming ionic compounds. Tin (ii or iv: learn the names/forumlas and charges, give the name of the positive )if the positive ion has more than 1 charge, include a roman numeral in the name, give the name of the negative ion.
