CHEM 1000H Chapter Notes - Chapter 7: Bohr Model, Magnetic Quantum Number, Radiography
Document Summary
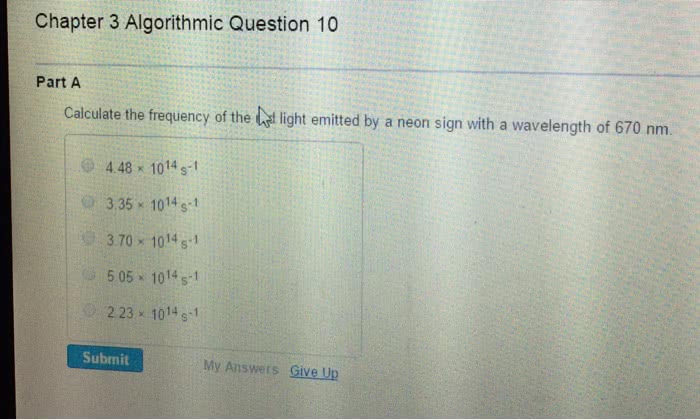
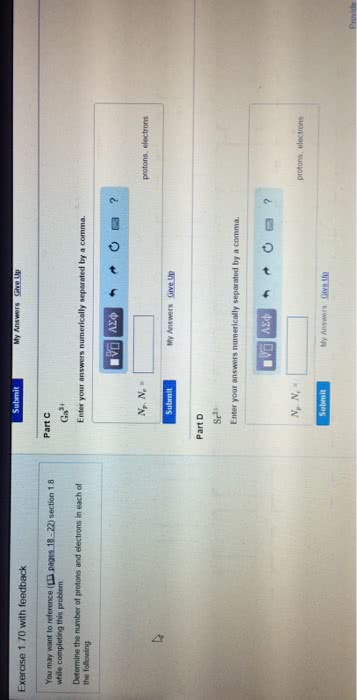
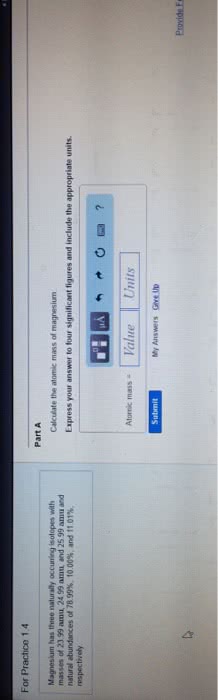
Chapter 7 the quantum-mechanical model of the atom: calculate the wavelength (in nm) of a the red light emitted by a neon sign with a frequency of 4. 74 1014. Hz: 633 nm, 158 nm, 142 nm, 704 nm, 466 nm. Answer: a: calculate the wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6. 88 1014. Hz: 229 nm, 436 nm, 206 nm, 485 nm, 675 nm. Answer: e: place the following types of electromagnetic radiation in order of increasing frequency. visible light microwaves. X-rays: microwaves < visible light < x-rays, x-rays < visible light < microwaves, microwaves < x-rays < visible light, x-rays < microwaves < visible light, visible light < x-rays < microwaves. Answer: b: which of the following visible colors of light have the largest frequency, green, red, blue, yellow, orange. Answer: c: which of the following visible colors of light have the longest wavelength, blue, green, yellow, red, violet.