VPHY 3100 Chapter Notes - Chapter 6-8: Extracellular Fluid, Integrin, Elastin
VPHY Notes
Ch 6 (Interactions between cells and the extracellular environment)
1. Water content of the body is split into what two compartments?
a. Intracellular compartment (67%)27-30L
b. Extracellular compartment (33%) 14-16.5L
i. 20% vascular fluid, blood plasma3.0-3.5L
ii. 80% as interstitial fluid 11-13L (non-vascular tissue fluid)
1. makes up the tissue fluid, found in the extracellular matrix
2. What two things is the cytoplasm (space between nucleus and plasma membrane) made up of?
a. Cytosol
i. Water based portion
ii. Fluid organelles reside in this
b. Organelles
i. Carry out cellular function
3. What function does the cell membrane serve?
a. Acts as a physical barrier between the cytoplasm and the extracellular fluid (ECF)
4. Living organisms are -% water by weight?
a. 70%
Extracellular environment
o Includes everything that lies outside of cells
o ECM (extracellular matrix)
▪ Gel-like hydrated material
▪ “Ground substance”amorphous gel-like substance surrounding cells
• Contains glycoproteins (proteins with side chains of sugars) such as integrins
o Integrinsclass of glycoproteins that extend from the cytoskeleton within a cell,
through its plasma membrane, and into the extracellular matrix
o Glue between cells and the extracellular matrix
o Join intracellular to extracellular compartments (Integrins help with signal relay)
▪ Contains Protein fibers
• Collagenmain structural protein in extracellular space
• Elastin lets tissues snap back to their original state after being stretched
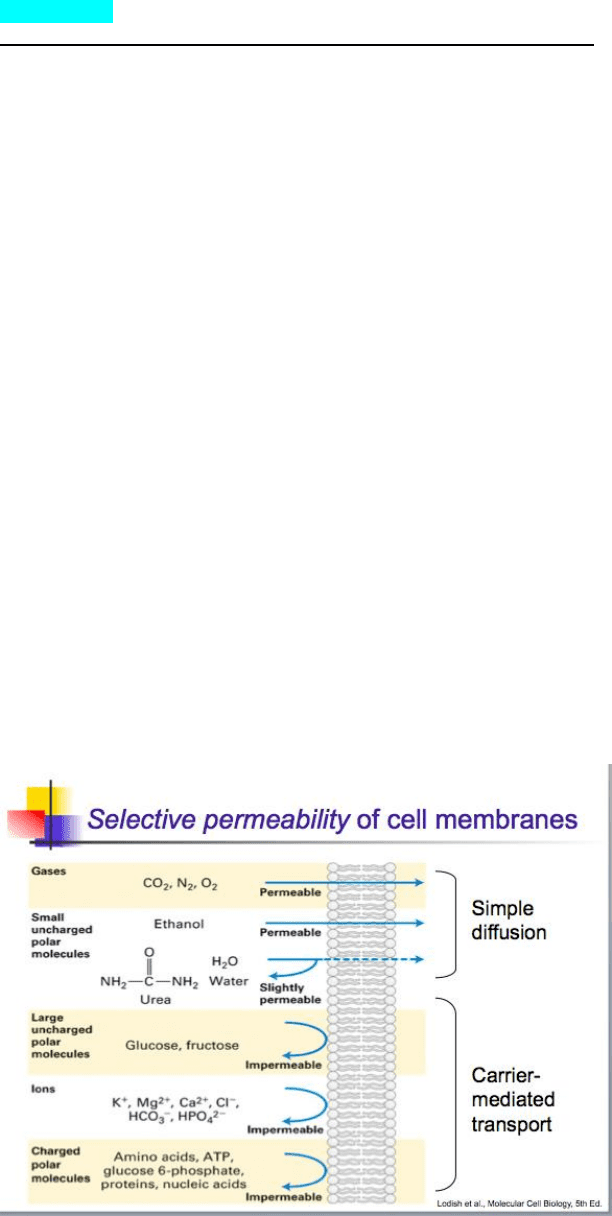
Selective permeability
Solutions and Diffusion
o Biological systems exist within aqueous solutions
▪ Solvent= water
▪ Solute= stuff dissolved in water
o Concentration
▪ Tells us the amount of solute relative to the amount of solution/solvent
find more resources at oneclass.com
find more resources at oneclass.com
▪ Concentration differences (gradients) lead to downhill net diffusion of solute from a region of
higher concentration to a region of lower concentration
• Gradient is a driving force
• Net diffusion this means that most is one direction but some still exists in the other
direction
o Simple Diffusion
▪ Diffusional driving force
• Proportional to the concentration gradient
• The degree to which a substance will diffuse across a lipid bilayer is dependent on the
selective permeability of that membrane
▪ Simple diffusion
• Non-carrier mediated, “downhill” movement of some molecules across a cell membrane
o Downhill-down the concentration gradient
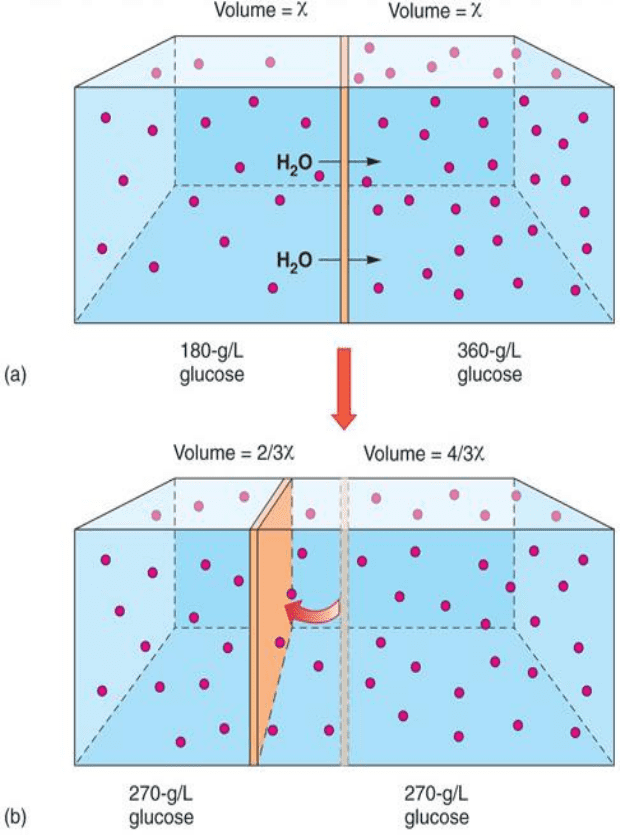
o Osmosis
▪ Net diffusion of water (solvent) across a membrane from regions of higher water concentration to
lower [H2O]
▪ Requirements
• Membrane must be selectively permeable to water
• Concentration gradient for the solute must exist across the membrane
• Solute must be osmotically active (membrane impermeable to solute)
• When water moves from high to low concentration (down gradient) a moveable partition
will move the opposite direction of movement to account for the increase in volume
▪ Net movement of water is towards the side with the most solute to even it out
▪
o Osmotic Pressure
find more resources at oneclass.com
find more resources at oneclass.com
▪ Force needed to counteract osmosis
▪ Increased solute concentrations increase the osmotic pressure of the solution
▪ Bar prevents volume change of water so adding more solute to one side would pull water to it
more strongly and lead to a higher osmotic pressure
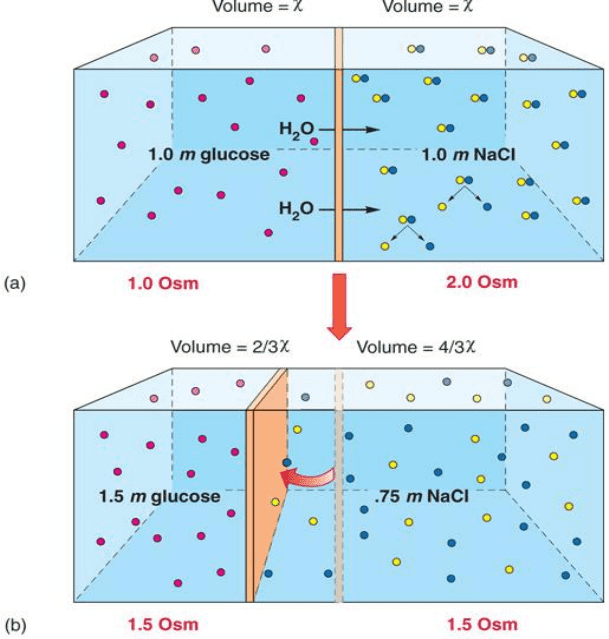
o Concentration Units
▪ Concentrationamount of solute relative to the amount of solution or solvent
• Molarity (M)
o 1 M soln = (1 mol solute)/ (1 L soln)
o 1 mole of glucose (180g) per liter solution= 1 molar
• Molality (m)
o 1 m soln = (1 mol solute)/ (1 kg solvent)
o 1 mole (180 grams glucose) per kilogram water= 1 molal
• Osmolality (Osm)
o Total molality of solution= sum of molalities of all solutes present
• One mole (mol) = 6.02E23 units
•
o Tonicity
▪ Total concentration of solutes
▪ Describes effect of a solution on the osmotic movement of water
▪ Differences in tonicity lead to osmotic movements of water
• Isotonic
o 2 solutions have same osmotic pressure across a semipermeable membrane
o Water can move across membrane can move across membrane freely without
changing the concentration of solutes on either side
o Out is balanced by in
• Hypotonic
o Solution that has a lower osmotic pressure than another solution
o Has less solute and more water than another solution
o Osmotically active
▪ Cells can burst in this scenario (hemolysis)
• Hypertonic
o Solution where the total molar concentration of all dissolved solute particles is
greater than that of another solution
o More solute, less water
o Ex: Seawater
find more resources at oneclass.com
find more resources at oneclass.com
Document Summary
Includes everything that lies outside of cells: ecm (extracellular matrix, gel-like hydrated material. Ground substance amorphous gel-like substance surrounding cells: contains glycoproteins (proteins with side chains of sugars) such as integrins. [na] concentration within the cell: the transport of na+ down its concentration gradient provides energy for glucose to be moved against the concentration gradient, resting cell, high [k+], low [na+] inside and the opposite outside. Movement via vesicles: some molecules are too large to enter or exit cells via carrier mediated transport, endocytosis (enter) 60 to -80 mv: vm always refers to the inside relative to the outside (assumes 0 mili moles on the outside) Inside, because it wants the inside of the cell to be closer to -88. Smooth muscles: connector neuron involved in an afferent and efferent loop. Insulation of peripheral axons: form myelin sheaths around axons of the cns, one oligodendrocyte per 15 internodes, myelin sheath (white matter, schwann cells (pns, function.

