CHEM103 Lecture Notes - Lecture 1: Trail Mix, Carbon Tetrachloride, Antoine Lavoisier
CHEM103 verified notes
1/10View all
Document Summary
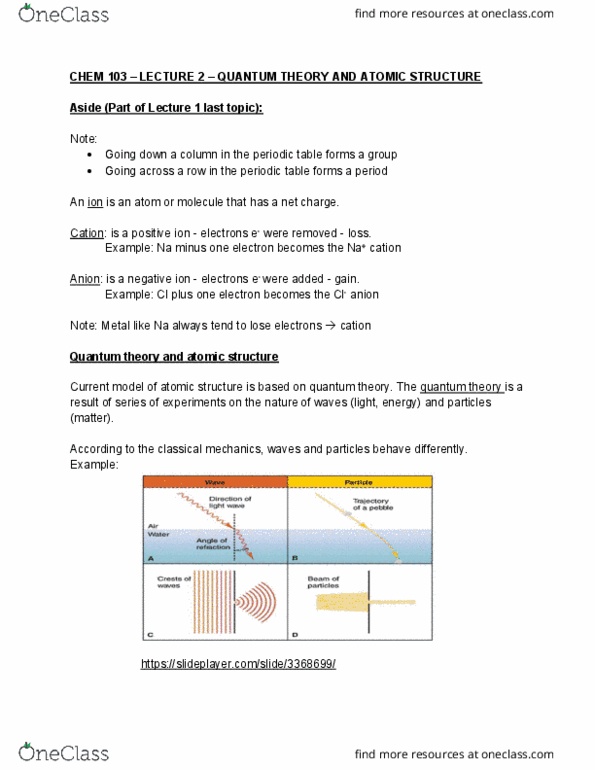
Chem 103 lecture 1 chemistry 30 review. Matter: everything that has weight and occupies space. Mixtures: two or more substances that are physically mixed. Example: air, trail mix, ocean water, limestone, blood, cosmetic formulations: law of conservation of mass lavoisier 1789. Mass is neither creator nor destroyed during chemical reaction: law of definite proportion proust 1799. A given compound contains exactly the same proportion of elements by mass. Example: ccl4 always has 1 c and 4 ci: law of multiple proportion dalton 1808. Two elements can combine together to form several different compounds. The relative number of atoms of each element in a given compound is always the same: chemical reactions only involve the rearrangement of atoms. Atoms are not created or destroyed during chemical reactions. Electrical discharge where made in a cathode tube. Conclusions: - negatively charged particles e- where seen.