1
answer
0
watching
251
views
23 Nov 2019
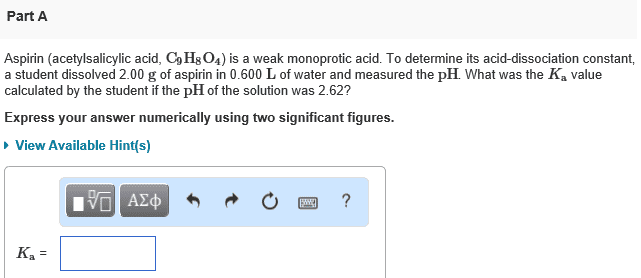
A typical aspirin tablet contains 324 mg of aspirin (C9O4H8), a monoprotic acid with a Ka= 2.8x10^-4. A monoprotic acid donates a single proton. If you dissolve two aspirin tablets in a 300 mL glass of water, what is the pH of the solution?
A typical aspirin tablet contains 324 mg of aspirin (C9O4H8), a monoprotic acid with a Ka= 2.8x10^-4. A monoprotic acid donates a single proton. If you dissolve two aspirin tablets in a 300 mL glass of water, what is the pH of the solution?
Nelly StrackeLv2
15 Nov 2019