CHM 132 Chapter Notes - Chapter 10: Irreversible Process, Spontaneous Process, Isothermal Process
Document Summary
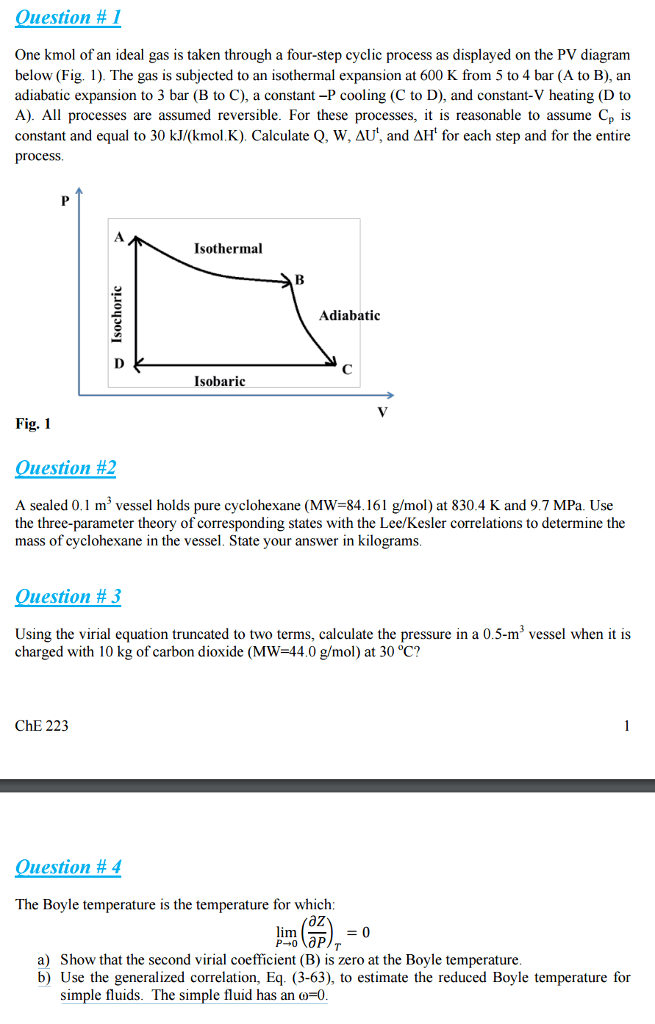
10. 2 the isothermal expansion and compression of an ideal gas. Isothermal process one in which the temperatures of the system and surroundings remain constant: e = 0 = q + w, q = -w. Expansion: one-step expansion no work called a free expansion, one-step expansion . The work performed to expand the gas is equal to the work performed on the weight. W = (m2)*g*h = -p v: two-step expansion. Work is not a state function therefore the work done in an one-step expansion and a two-step expansion will not be the same. If drawn as a p v. v graph, then the work done can be find as area. W continues to increase as the number of steps (n) increases. |wn| = n limiting case i=1 pi vi. With infinite number of steps, the external pressure is always. V1 pexdv almost exactly equal to the pressure produced by the gas.