CHEM1201 Lecture Notes - Lecture 20: Ambient Pressure, Barometer, Gas Laws
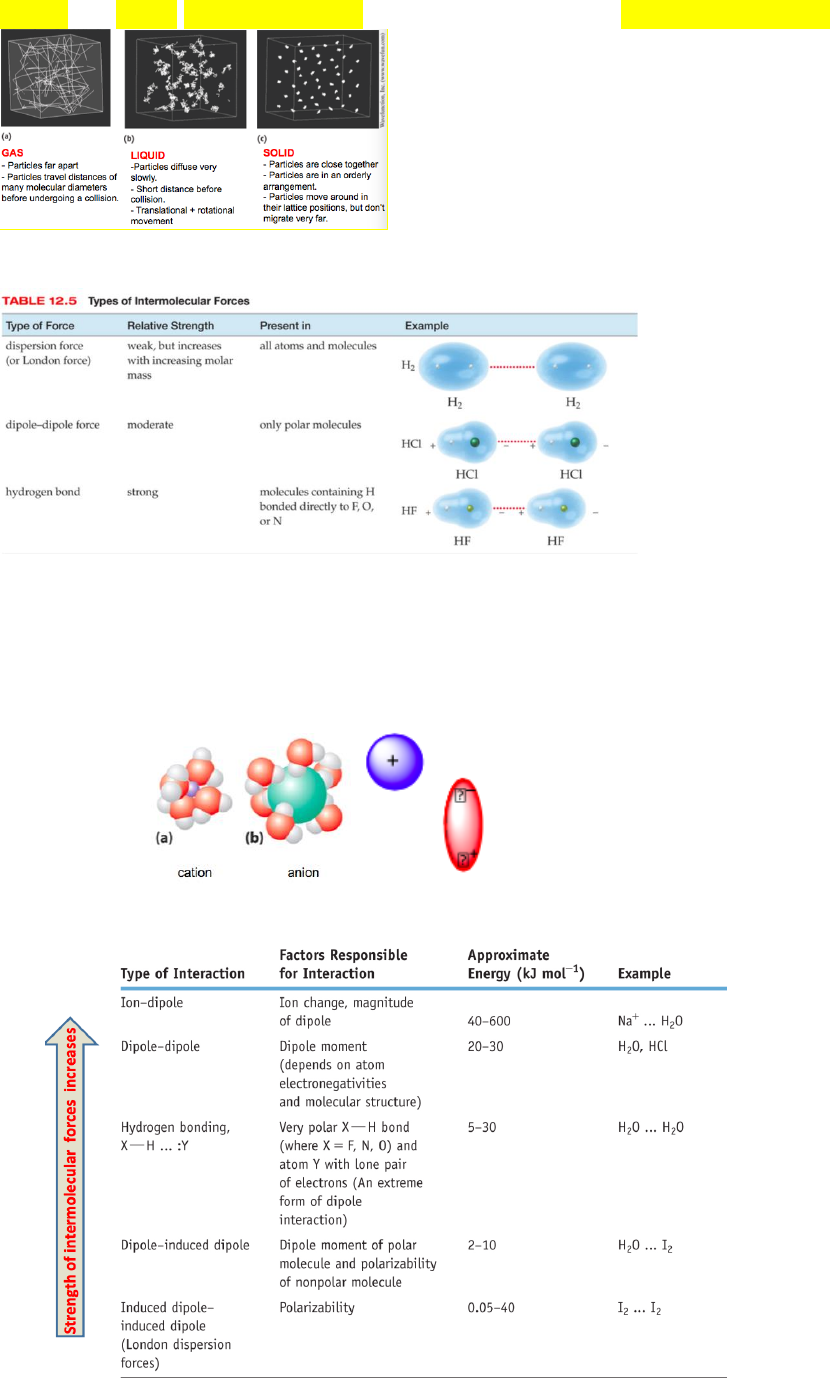
States of matter:
- Gases
- Liquids
- Solids
Liquids and solids (condensed phases) are formed as a result of intermolecular forces
Types of intermolecular forces:
Additional types of intermolecular forces:
- Ion-dipole forces:
o E.g. a solution of a salt in water
o Electrostatic attraction between the charge on an ion and a dipole (of a
solvent)
o Note the orientation of the water molecules toward cations and anions
o
- dipole-induced dipole forces
o attraction between a dipolar molecule and a polarisable molecule
find more resources at oneclass.com
find more resources at oneclass.com

Intermolecular interaction vs. thermal energy:
- in all three states of matter, there is competition between intermolecular forces and
thermal movement of the particles
- Gases:
o Intermolecular interactions weak, thermal movement overcomes
intermolecular interaction
- Liquids:
o Intermolecular interactions keep the particles together, but thermal
movement leads to chaotic motion
- Solids:
o Intermolecular interactions too strong to be overcome by thermal movement
The stronger the intermolecular forces, the higher the boiling point.
Properties of gases, liquids, and solids:
- Gases:
o Readily compressible
o Rapid expansion to fill the available space; freedom of particle movement
o Collection of widely spaced molecules or atoms which are in chaotic motion
o Indefinite shape and volume (container required to keep them into a
confined space)
- Liquid:
o Difficult to compress
o Molecular movement; particles not fixed at specific locations
o Flows; takes the form of the container it is kept in
o Indefinite shape, fixed volume
o Collection of closely spaced molecules or atoms with short-range order
- Solids:
o Very difficult to compress, well defined shape
o Molecules or atoms at fixed position
o Definite shape, fixed volume
o Collection of closely spaced molecules of atoms with mostly long-range order
(except when solids are amorphous)
Differences and similarities between gases:
- Different gases have different uses, natural abundance, chemical reactivity, and
interaction with electromagnetic radiation
- Different gases show almost universal thermodynamic properties under conditions
near ambient pressure and temperature
-
- The volume of one mole is virtually identical for all gases and amounts to 22.4 Lmol-1
at 0oC and 1 atm. This is called the molar volume
find more resources at oneclass.com
find more resources at oneclass.com
Document Summary
Liquids and solids (condensed phases) are formed as a result of intermolecular forces. Ion-dipole forces: e. g. a solution of a salt in water, electrostatic attraction between the charge on an ion and a dipole (of a solvent, note the orientation of the water molecules toward cations and anions. Dipole-induced dipole forces: attraction between a dipolar molecule and a polarisable molecule. Intermolecular interaction vs. thermal energy: in all three states of matter, there is competition between intermolecular forces and thermal movement of the particles. Intermolecular interactions weak, thermal movement overcomes intermolecular interaction. Intermolecular interactions keep the particles together, but thermal movement leads to chaotic motion. Intermolecular interactions too strong to be overcome by thermal movement. The stronger the intermolecular forces, the higher the boiling point. Gases: readily compressible, rapid expansion to fill the available space; freedom of particle movement, collection of widely spaced molecules or atoms which are in chaotic motion.

