PHYS 2108 Lecture Notes - Lecture 2: Kilogram, Standard Deviation, Analog Devices
2-1
LAB 2:
Measurement of Mass, Length, and Time
Coside the folloig stateet: Bee osts te.
Ten what? If e’e talkig aout a ee fo te dollas, it’s a little steep. Te Euos, it’s a lot steep. Ten
pesos? Pass. The problem is compounded when we realize the amount of beer is also unknown. If we
settle on $10, questions remain about this deal if it’s for a single draw, a can, a case or a keg.
Anything that can be counted has a unit. six-pack costs ten dollars. Without a uit, easued
values are meaningless. After all, the point of a measurement is to tell us how much of something we
have. Different systems of units exist around the world. You are probably most familiar with the
English System of feet for distance and pounds for force. The metric system or MKS (meter, kilogram,
seconds) is another system used globally for science and is part of the International System (SI) of units.
Four fundamental units—length, time, mass and charge—are not the only physical quantities, but they
are the most basic. Velocity (m/s), Acceleration (m/s2), Force (N or kg m/s2), Energy (J or kg m2/s2) are
real physical quantities, but they are made (derived) from relationships between the most basic units.
Because the units for these derived quantities can sometimes get to be large and cumbersome, we give
them special names like Newtons (N) or Joules (J). Anytime you must perform a calculation, *always*
include the units in your work and break them down to their most basic forms if necessary to cancel any
out. Units in calculations can always signal when something might be wrong, such as a velocity that
comes out in hours per miles because we performed the calculation upside down!
Most of the time when reporting measurements we will do so in standard units (m, kg, s). If
measurements are small, they will often be recorded in smaller units (cm, mm, g, etc.). It is important to
know how the metric prefixes modify the base units, and to be able to convert them accordingly.
Prefix
Modification to Base Unit
Example
pico- (p)
× 10-12 or ÷ 1 trillion
0.3pF = 3 × 10-13F
nano- (n)
× 10-9 or ÷ 1 billion
532nm = 5.32 × 10-7m
micro- μ or u
× 10-6 or ÷ 1 million
3.6μm = 3.6 × 10-6m
milli- (m)
× 10-3 or ÷ 1,000
256ms = 0.256s
centi- (c)
× 10-2 or ÷ 100
2.5cm = 0.025m
kilo- (k)
× 103 or × 1,000
78.5g = 0.0785kg
mega- (M)
× 106 or × 1 million
500MJ = 5 × 108J
giga- (G)
× 109 or × 1 billion
8.21GW = 8.21 × 109W
tera- (T)
× 1012 or × 1 trillion
2TB = 2 × 1012B
Special Note: Even though kg are not the base unit, in SI the kg is the stadard uit. It is a ystery.
find more resources at oneclass.com
find more resources at oneclass.com
2-2
Unit Conversions
All systems have something in common: they measure the same physical quantities, but the standard
aout a a fo sste to sste. Beause diffeet uits of distae still easue distance,
you can easily convert from one system to another if you know the conversion factor a ehage
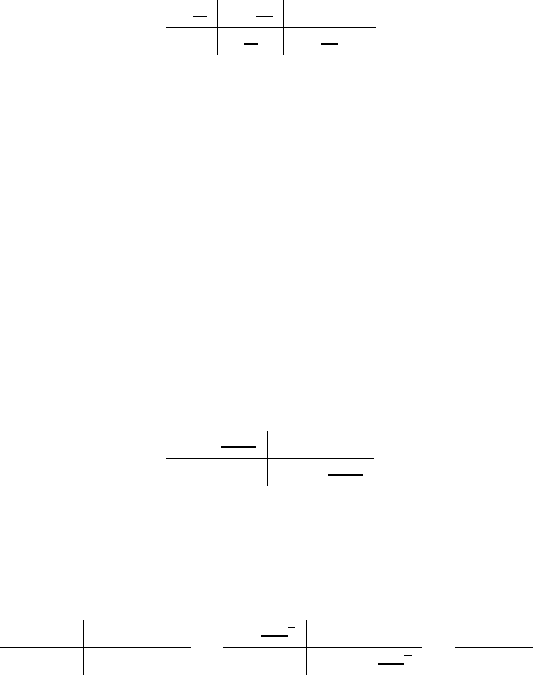
ate etee sstes. Consider a conversion of 1 ft to cm knowing 1 in = 2.54 cm
1 ft
12 in
2.54 cm
=
30.48 cm
ft
in
I the oesio aoe, egi ith the aout eedig oesio. Beause e do’t ko the
conversion factor from cm to ft, we will need to use a second conversion to go from ft to in, then inches
to cm. Multiply the original value by the conversion factor in a way that makes sense (this means it is
not 1 ft × 1 ft, instead we use 1 ft × 12 inches / ft). Use the second conversion factor next. This hat
method is a convenient way to keep track of conversions without using a large number of ( ) × ( ) × ’s.
Once all the necessary conversion factors are charted up, cancel units that match on top and bottom.
Multiply across the top and multiply across the bottom. Simplify the result if it remains a fraction.
More often than not, your conversions will be within the same unit system (from one prefix to another).
Because the decimal system is based in tens, it is very easy to work with because we can simply shift the
decimal point left or right. For example, convert 34.6 mm to m. Our intuition tells us to move the
decimal point three places to the left eause it’s diided , fo a esult of . . Chek it.
34.6 mm
1 m
=
0.0346 m
1000 mm
A common mistake students make is to use this logi o aeas, olues, o athig that’s suaed. Fo
example, convert 36 cm2 to m2. An easy trap to fall into is to move the decimal two places and call it
0.36m2. Let’s explore that.
36 cm2
(1 m)2
=
36 cm2
1 m2
=
36 m2
=
0.0036 m2
(100 cm)2
10000 cm2
10000
Whe the oesio happes fo a suae, it happes tie: oe fo the legth ad oe fo the
idth. This logi applies to a suaed uatit, ee if suaed-tie as a unit is hard for us to
understand. Because the math is much more difficult to remember to do mentally, it is a good idea to
do any unit conversions before plugging them into squares. The same mentality applies to volumes and
anything raised to a power.
find more resources at oneclass.com
find more resources at oneclass.com

2-3
Developing a Sense for Units
Unless you come from anywhere north, south, east or west of the United States, you probably grew up
with the English System of units (inches, feet, pounds) and have no instinct about the metric system (or
SI). Science is a global community and it is always conducted in SI, so American students can find
theseles at a disadatage eause e do’t hae a good sese of hat the uits mean.
The point of doing unit analysis is as a safeguard for errors. Bad units will tell you a calculation was done
incorrectly (the hr/mi example above), but they also mean to give us sense of scale for our results. Work
on trying to visually translate a value into units you understand, and compare that to your life
epeiee to let ou gut feelig ifo ou aout a ase. Fo the example above,
36 cm2 looks like a 6 cm by 6 cm square.
There are about 2 ½ cm in an inch, or a cm is about the width of a finger.
A 6×6cm square is about 2 ½ in × 2 ½ in, or about the size of the palm of your hand.
A meter is a little more than 3 feet, so a square meter is a little more than a square yard or
about half of the front face of a refrigerator.
0.36m2 is about 1/3 of that.
This is nowhere near the size of the palm of your hand. The conversion was done incorrectly
and 36cm2 ≠ .2.
Special Considerations in Measurement
Accuracy and Precision:
These words may sometimes be used interchangeably in English, but they are distinctly different in
scientific language.
Precision refers to how close a measurement can get. A measurement device with higher precision
can read out to more decimal places. By simply looking at two measurements, 0.1 seconds and
0.0378 seconds, we have an idea of the implied precision of the two devices used to take the
measurements. The smallest increment explicitly measurable by a device is called its least-count.
Precision efes to the loseess of a easueet. Instrument precision is related to its least-
count, but we can also talk about the precision of a collection of measurements. Experimental
precision can refer to how closely grouped together a collection of measurements are to one
another. In both cases, highe peisio iplies less iggle oo.
Accuracy refers to how close a measurement is to the true value or a generally accepted value. If I
use a thermometer to measure the temperature of boiling water at sea level, and I find the
temperature to be 94.238° C, the thermometer appears to be very precise but not accurate because
ate’s oilig poit is atuall ° C at sea leel. Whe e calibrate an instrument, it means we
confirm its accuracy by adjusting the reading until it matches a comparison standard with a known
value.
Accuracy and precision are different from one another, and the best measurements have both accuracy
and precision. The two do not always appear simultaneously. A target makes a good analogy for
understanding precision (closeness) and accuracy (correctness).
find more resources at oneclass.com
find more resources at oneclass.com
Document Summary
Co(cid:374)side(cid:396) the follo(cid:449)i(cid:374)g state(cid:373)e(cid:374)t: (cid:862)bee(cid:396) (cid:272)osts te(cid:374). (cid:863) If (cid:449)e"(cid:396)e talki(cid:374)g a(cid:271)out a (cid:271)ee(cid:396) fo(cid:396) te(cid:374) dolla(cid:396)s, it"s a little steep. The problem is compounded when we realize the amount of beer is also unknown. If we settle on , questions remain about this deal if it"s for a single draw, a can, a case or a keg. Anything that can be counted has a unit. (cid:862)(cid:1005) six-pack costs ten dollars. (cid:863) without a u(cid:374)it, (cid:373)easu(cid:396)ed values are meaningless. After all, the point of a measurement is to tell us how much of something we have. Different systems of units exist around the world. English system of feet for distance and pounds for force. The metric system or mks (meter, kilogram, seconds) is another system used globally for science and is part of the international system (si) of units. Four fundamental units length, time, mass and charge are not the only physical quantities, but they are the most basic.

