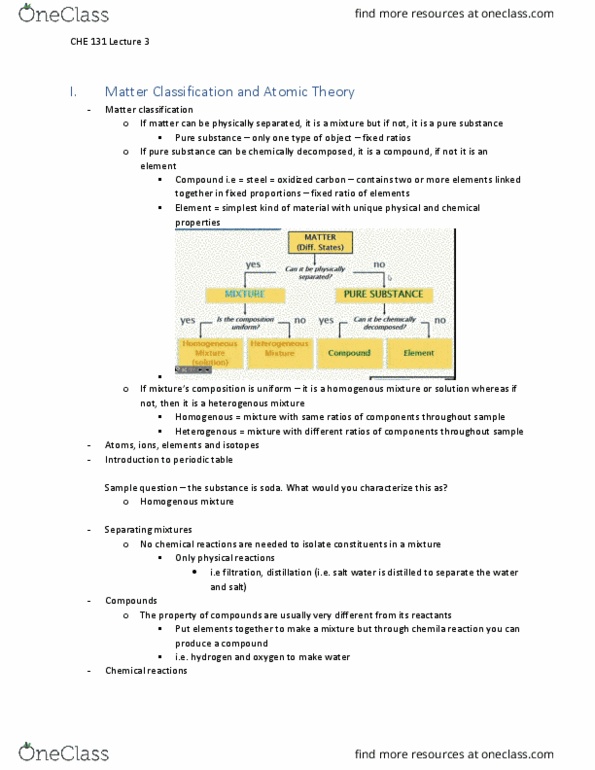
Chromatography: Separating Mixtures
Introduction:
Magic marker inks are often mixtures of several compounds. Paper chromatography is a common method of separating various components of a mixture. After separation, you can observe the different colors that make up a particular color of magic marker ink.
You can also calculate a ratio Rf, which compares how far each compound traveled to how far each solvent (substance that dissolves another substance) traveled during the experiment.
Rf = Ds/Df
Ds = Distance traveled by the compound
Df = Distance traveled by the solvent
Materials:
coffee filter, tape, isopropyl alcohol (rubbing alcohol), water, 3 different color magic markers (not permanent); 3 identical tall, narrow drinking glasses; metric ruler, 3 pencils
Pre-lab Questions:
List one extensive and one intensive property of marker ink.
Intensive: Amount Doesn't Matter
Extensive: Amount Matters
An extensive property of marker ink would be volume or ink.
An intensive property of marker ink would be the color of ink.
Define mixture.
A combination of two or more substances that are not chemically combine with one another.
Is tap water a heterogeneous or homogenous mixture? Explain your answer.
Tap water is a homogeneous mixture. I think this because I can see that it has uniform. It doesnât contain any different molecules as well.
Is isopropyl alcohol (rubbing alcohol) a heterogeneous or homogenous mixture? Explain your answer.
I believe rubbing alcohol is a homogeneous mixture. I think this because I can physically see that it has uniform. It also stays in the same phase.
Procedure:
1. Cut 3 strips of coffee filter. Be sure that the strips are much narrower and slightly taller than the drinking glasses.
2. In the center the strips, about 3 cm from one end, place a dot of the marker to be tested. The dots should be about 0.2 cm in diameter and dark enough to be clearly visible.
3. Place about 2 cm of water in each glass.
4. Tape the unmarked end of each strip to the center of a pencil so that the strip hangs down when the pencil is held horizontally.
5. Carefully insert the strips into the glasses, dotted end down. The dot must be above the water, and the sides of the coffee filters cannot touch the sides of the glasses.
6. Let the setup stand for 20 minutes. Follow procedure 8 as your set up stands.
7. Record your observations after 20 minutes in the table below. Measure Df (in cm) for the solvent. Measure the Ds (in cm) for each color that you see on the coffee filter (there may not be three). Calculate the Rf for each color using the equation in the Introduction section.
8. Repeat steps 1â7, except place about 2 cm of isopropyl alcohol (rubbing alcohol) in each glass instead of water.
9. Clean up your work area.
Data:
Solvent: water
Color 1: Green
Color 2: Purple
Color 3: Yellow
Solvent: isopropyl alcohol (rubbing alcohol)
Color 1: Green
Color 2: Purple
Color 3: Yellow
Post-Lab Questions:
5. What evidence is there that marker ink is a mixture?
The marker ink is a mixture. The evidence is when the marker ink separated.
6. Each compound in the marker ink is represented by a color. Did one compound travel farther than the others? Explain why you think that this is the case.
Yes, I found out that the darker of the three colors traveled further.
7. Did the compounds travel farther in the water or the isopropyl alcohol (rubbing alcohol)? Explain why you think that this is the case.
The compounds traveled faster in the water than the rubbing alcohol. I think the reason why is because water is smaller on a molecular level versus rubbing alcohol. So it is easier for the dyes to separate.
8. How could you improve upon the accuracy of your Rf measurements?
I feel that accuracy could be improved if we changed the scale measurement to millimeters (mm) instead of cm (centimeters).
Water traveled faster than the rubbing alcohol. I personally think it has to do with something like the size of the molecule like the smaller the molecule the easier it is.