CHEM 102 Lecture Notes - Lecture 23: Phosphorus Pentachloride, Inert Gas, Exothermic Process
Document Summary
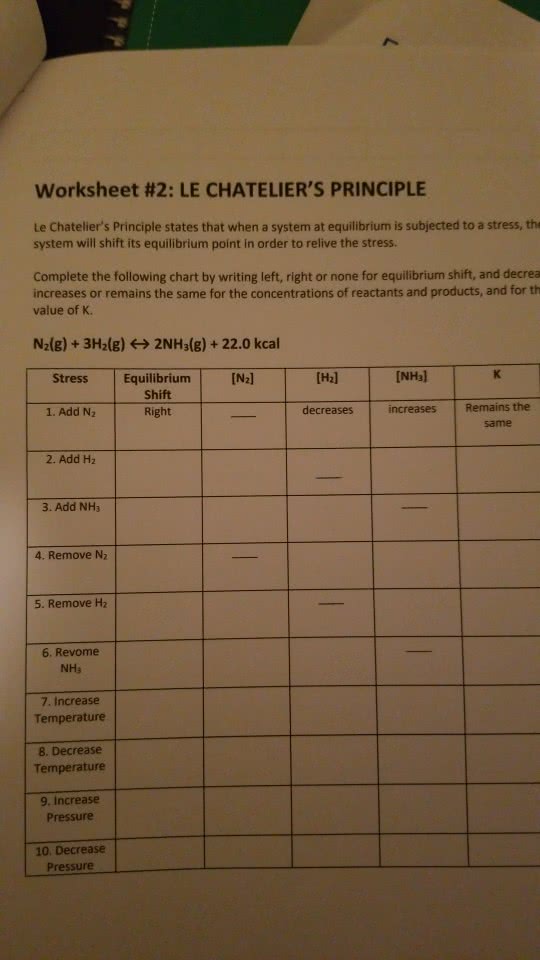
If a change (or stress) is applied to a reaction @ equilibrium, the reaction will shift in the d change/stress. Fe3+ (aq) + scn- (aq) fe(scn)2+ (aq) Add scn- or fe3+ ---> shifts to the right. Remove scn- or fe3+ ---> shifts to the left. As p increases (or v descreases) --> reaction will shift to decrease the pressure (s with fewer gas molecules) As p decreases (or v increases) --> reaction will shift to increase the pressure (sh more gas molecules) Predict the shift in equilibrium positions that will occur when the volume is reduced. 6 moles 0 moles shifts to the right. Pcl5 (g) pcl3 (g) + cl2 (g) 1 mole 2 moles shifts to the left. Addition of an inert gas (he, ne, ar, n2, sf6, etc. ) can effect the pressure and conce and products. Volume expands --> partial pressure change --> shift in response. No volume change --> no partial pressure change --> still @ equilibrium.