1
answer
0
watching
326
views
11 Dec 2019
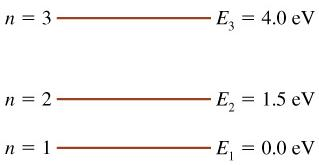
The figure is an energy-level diagram for a simple atom.
What wavelengths appear in the atom's emission spectrum?
#1: From wavelength 3 to 1
#2: From wavelength 3 to 2
#3: From wavelength 2 to 1
What wavelengths appear in the atom's absorption spectrum?
#5: From wavelength 1 to 2
#6: From wavelength 1 to 3

The figure is an energy-level diagram for a simple atom.
What wavelengths appear in the atom's emission spectrum?
#1: From wavelength 3 to 1
#2: From wavelength 3 to 2
#3: From wavelength 2 to 1
What wavelengths appear in the atom's absorption spectrum?
#5: From wavelength 1 to 2
#6: From wavelength 1 to 3
Supratim PalLv10
20 Sep 2020


